tft lcd glass substrate free sample
Be described the composition that can contain in glass formula of the present invention below, the containing ratio of each composition represents with weight percent.
2content is higher, and chemical resistant properties and physical strength can increase, and strain point increases, and the high temperature viscosity of glass increases, SiO
2content is lower not easily forms glass, and strain point declines, and the coefficient of expansion increases, and acid resistance declines, and alkali resistance declines.SiO
3chemical durability of glass can be improved and reduce devitrification of glass tendency, improve the composition of Young"s modulus simultaneously.Al
3content is lower, the easy crystallization of glass, physical strength is lower be unfavorable for shaping.The present invention introduces the Al of 14-22wt%
3being found performance for improving, reducing glass viscosity.On the other hand reduce proportion, improve resistance to BHF, improve glass meltability, make glass not easily devitrification and low expansion composition can be subtracted, be required composition.The present invention introduces the B of 3-12wt%
MgO is conducive to founding of glass, improves the stability of glass, suppresses devitrification of glass tendency, and MgO has when not reducing strain point and reduces high temperature viscosity, makes glass be easy to the feature melted.The present invention have chosen 5-10wt%MgO, preferred 6-9wt%MgO.
The molar content of SrO is 0-5%, and as fusing assistant and prevent glass from occurring crystallization, improve the specific refractory power of glass, if content is too much, glass density can be too high, causes the quality of product overweight.So the content of SrO is defined as 1-3%.
3, or yttrium oxide or cerium oxide or Samarium trioxide or Erbium trioxide can significantly improve Young"s modulus and the strain point of glass, the temperature of fusion of glass can be reduced simultaneously.With lanthanum trioxide La
Concrete steps: weigh each oxide compound according to each embodiment listed in table 1, table 2, fully mix, make it even, are poured into by compound in crucible, be incubated 8-12h at lower 1610 DEG C-1640 DEG C of High Temperature Furnaces Heating Apparatus.In order to make glass ingredient even, platinum rod under high temperature, is used to stir.The glass metal melted is cooled to shaping required temperature range, produces the thickness of the glass substrate that flat-panel screens needs, simpler cold work is carried out to shaping glass substrate, finally the Basic Physical Properties of glass substrate is tested.The characteristic datas such as the boron-containing quantity of glass, glass yield, thermal expansivity, strain point, liquidus temperature, high temperature viscosity, Young"s modulus, density, optical transmittance can be obtained respectively.
The technical measurement that glass property listed by embodiment in table 1, table 2 is commonly used according to glass art.Thermal linear expansion coefficient adopts horizontal expander instrument to measure, and the thermal linear expansion coefficient (CTE) within the scope of 50-350 DEG C is with × 10
3; High temperature viscosity adopts drum type brake to rotate high temperature viscosimeter and measures, and utilizes VFT formulae discovery temperature of fusion, and unit is DEG C (temperature when temperature of fusion refers to that glass melt viscosity reaches 200 pool); Liquidus temperature adopts normal gradients stove to measure, and unit is DEG C; Young"s modulus adopts resonant method test; Transmitance adopts ultraviolet-visible spectrophotometer test, and unit is %.
As can be seen from table 1, table 2, can be proved again by each embodiment: the glass substrate produced by means of glass ingredient principle provided by the invention can reach following technical indicator after testing: the thermal linear expansion coefficient (CTE) within the scope of 50-350 DEG C is 30-37 × 10
3; High temperature viscosity adopts drum type brake to rotate high temperature viscosimeter and measures, and utilizes VFT formulae discovery temperature of fusion for being greater than 1619 DEG C (temperature when temperature of fusion refers to that glass melt viscosity reaches 200 pool); Young"s modulus adopts resonant method test for being greater than 83 GPa; Light transmission rate adopts ultraviolet-visible spectrophotometer test for being greater than 92%.

The present invention relates to a glass composition, and more particularly to a glass composition suitable for a glass substrate for a flat panel display (FPD) such as a liquid crystal display (LCD). The present invention also relates to an FPD glass substrate using this glass composition, a flat panel display, and a method of producing a glass substrate for a flat panel display.
There has been an increasing demand for flat type image display apparatuses called “flat panel displays (FPDs)” such as liquid crystal displays (LCDs). Among the FPDs, active matrix LCDs using thin film transistors (TFTs) have been widespread because they display high quality images. In an active matrix LCD, a TFT circuit is formed on the surface of a glass substrate. Conventionally, the step of forming a TFT circuit on the surface of a glass substrate is carried out in an environment of 1000° C. or higher. In recent years, however, a low-temperature polysilicon (p-Si) active matrix LCD, in which a TFT circuit can be formed at a temperature of 500 to 600° C., has been developed. This development makes it possible to use not only silica glass having stable physical properties under high temperature conditions but also aluminosilicate glass and aluminoborosilicate glass as glass substrates for LCDs.
An FPD glass substrate is required to have a small thickness and a very smooth surface. In addition, there is a strong demand for the production of larger glass substrates in response to a recent increase in the size of FPDs. There are various methods of producing glass substrates, and among them, a downdraw process is the best method for obtaining such glass substrates efficiently. In the downdraw process, molten glass is fed into a trough formed in the upper part of a glass sheet forming apparatus, and the molten glass flowing over the both edges of the trough is allowed to flow downward along the outer wall of the forming apparatus. Then, two streams of molten glass are fused together at the lower end (root) of the forming apparatus so as to produce a single glass ribbon continuously. After the glass ribbon solidifies, it is cut into pieces of a desired size. Thus, glass substrates are obtained.
Compared with a float process, which is another method for producing glass substrates, the downdraw process has a drawback in that glass substrates are susceptible to devitrification because they are formed at a lower temperature. Therefore, a glass composition having a low devitrification temperature is required in order to produce a glass substrate stably by the downdraw process. In addition, in order to form a TFT circuit on a glass substrate stably, a glass composition is required to have high thermal stability (for example, a high glass transition temperature or a high strain point).
JP 2006-169107 A discloses an aluminosilicate glass composition that can be produced by a method other than the downdraw process. This aluminosilicate glass composition is substantially free of alkali metal oxides, and consists essentially of, in terms of mass %: 60 to 67% of SiO2; 16 to 23% of Al2O3; 0 to 15% of B2O3; 0 to 8% of MgO, 0 to 18% of CaO, 0 to 15% of SrO, and 0 to 21% of BaO. The total content of MgO, CaO, SrO, and BaO is 12 to 30%. This composition is not, however, suitable for the downdraw process. Furthermore, as shown in Examples, this composition has a high content of BaO, which is not desirable in view of its environmental load and production cost.
JP 3988209 B2 discloses a glass composition suitable for a glass substrate for an FPD. This glass composition is substantially free of alkali metal oxides, and can be formed by the float process. This composition is not, however, suitable for the downdraw process because it has a high devitrification temperature of 1250° C. as shown in Examples.
JP 2009-013049 A discloses a glass composition being free of alkali metal oxides, As2O3, and Sb2O3, containing, in terms of mol %, 55 to 75% of SiO2, 7 to 15% of Al2O3, 7 to 12% of B2O3, 0 to 3% of MgO, 7 to 12% of CaO, 0 to 5% of SrO, 0 to 2% of BaO, 0 to 5% of ZnO, and 0.01 to 1% of SnO2, having a liquidus viscosity of 105.2dPa·s or higher, and having a temperature of 1550° C. or lower at a high temperature viscosity of 102.5dPa·s.
BaO, which is one of the glass components, is known to have effects of suppressing the phase separation of glass, improving the meltability, and decreasing the devitrification temperature (see paragraph 0023 of JP 3988209 B2). However, BaO has a high environmental load and its raw material is expensive, which results in an increase in the production cost of a glass substrate. Therefore, glass compositions substantially free of BaO are needed. SUMMARY OF THE INVENTION
It is an object of the present invention to provide a glass composition being suitable for an FPD glass substrate, having high thermal stability, being substantially free of BaO but having a low devitrification temperature, and being suitable for the production of a glass substrate by the downdraw process.
The glass composition of the present invention contains, in terms of mass %: 54 to 62% of SiO2; 4 to 11% of B2O3; 15 to 20% of Al2O3; 2 to 5% of MgO; 0 to 7% of CaO; 0 to 13.5% of SrO; 0 to 1% of K2O; 0 to 1% of SnO2; and 0 to 0.2% of Fe2O3. This glass composition is substantially free of BaO, the total content of alkaline earth metal oxides (MgO+CaO+SrO) is 10 to 18.5 mass %, and the devitrification temperature of the glass composition is 1200° C. or lower.
In the production method of the present invention, a melt of the glass composition of the present invention is formed into a glass substrate for an FPD by a downdraw process.
The glass composition of the present invention is suitable for a glass substrate for an FPD. This glass composition has high thermal stability. This glass composition has a low devitrification temperature even though it is substantially free of BaO. This glass composition is suitable for the production of a glass substrate by the downdraw process. BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a graph showing the dependence of the devitrification temperature of the glass composition of the present invention on the balance between the content of CaO and the content of SrO. In FIG. 1, the devitrification temperature is plotted as a function of the percentage (CaO/ROs) of CaO in all the alkaline earth metal oxides (ROs).
FIG. 2 is a graph showing the dependence of the devitrification temperature of the glass composition of the present invention on the balance between the content of CaO and the content of SrO. In FIG. 2, the devitrification temperature is plotted as a function of the percentage (SrO/ROs) of SrO in all the alkaline earth metal oxides (ROs).
FIG. 5 is a graph showing the linear thermal expansion coefficient of the glass composition of the present invention as a function of the content of K2O.
The reasons for determining the composition of the glass composition of the present invention are described below. In the following description, the unit “%” which expresses the contents of the components denotes “mass %”.
SiO2is a component for forming a glass skeleton, and has an effect of increasing the chemical durability and heat resistance of the glass. When the content of SiO2is less than 54%, such an effect cannot be obtained sufficiently. On the other hand, when the content of SiO2is more than 62%, the devitrification temperature of the glass increases. Furthermore, the melt viscosity of the glass increases as the meltability thereof deteriorates, which makes it difficult to form a glass substrate by the downdrawn process. Therefore, the SiO2content is 54% or more, preferably 55.5% or more, and more preferably 56.5% or more, focusing on its lower limit. The SiO2content is 62% or less, preferably 60% or less, and further preferably less than 58.4%, focusing on its upper limit. The content of SiO2is 54% or more and 62% or less, preferably 55.5% or more and 60% or less, and more preferably 56.5% or more and 58.4% or less.
B2O3is a component for decreasing the viscosity of glass and promoting the melting and refining of the glass. When the Content of B2O3is Less than 4%, the meltability of the glass deteriorates, which makes it difficult to form a glass substrate by the downdraw process. On the other hand, when the content of B2O3is more than 11%, the volatilization of B2O3from the surface of the molten glass increases, which makes it difficult to homogenize the glass. Therefore, the B2O3content is 4% or more, preferably 7% or more, and more preferably 8% or more, focusing on its lower limit. The B2O3content is 11% or less, and more preferably 10% or less, focusing on its upper limit. The content of B2O3is 4% or more and 11% or less, preferably 7% or more and 11% or less, and more preferably 8% or more and 10% or less.
Al2O3is a component for forming a glass skeleton, and has an effect of increasing the strain point of glass. A glass substrate used for a polysilicon (p-Si) LCD is required to have high thermal stability when a TFT circuit is formed thereon at a temperature of 500 to 600° C. Therefore, Al2O3, which has an effect of increasing the strain point of glass, is an important component for the glass composition of the present invention. When the content of Al2O3is less than 15%, the strain point of the glass decreases, and as a result, a glass composition suitable for a glass substrate for an FPD is not obtained. On the other hand, when the content of Al2O3is more than 20%, the acid resistance of the glass decreases, and the glass cannot withstand the acid treatment step in the production of FPDs, for example. Therefore, the Al2O3content is 15% or more, preferably 16% or more, and more preferably 18% or more, focusing on its lower limit. The Al2O3content is 20% or less, focusing on its upper limit. The content of Al2O3is 15% or more and 20% or less, preferably 16% or more and 20% or less, and more preferably 18% or more and 20% or less.
MgO is a component for decreasing the viscosity of glass and promoting the melting and refining of the glass. MgO further has an effect of decreasing the density of glass, and therefore it is effective in reducing the weight of the resulting glass and improving the meltability thereof. When the content of MgO is less than 2%, the meltability of the glass deteriorates, which makes it difficult to form a glass substrate by the downdraw process. On the other hand, when the content of MgO is more than 5%, the glass phase separation develops and thereby the acid resistance thereof decreases. As a result, the glass cannot withstand the acid treatment step in the production of FPDs, for example. Therefore, the MgO content is 2% or more, and preferably 3% or more, focusing on its lower limit. The MgO content is 5% or less, focusing on its upper limit. The content of MgO is 2% or more and 5% or less, and preferably 3% or more and 5% or less.
CaO is a component for decreasing the viscosity of glass and promoting the melting and refining of the glass. The glass composition of the present invention does not necessarily need to contain CaO, but preferably it contains CaO to improve the meltability of the glass and stabilize the production of a glass substrate by the downdraw process. Furthermore, a proper balance between the CaO content and the SrO content in the glass composition of the present invention allows the glass composition to have a lower devitrification temperature while maintaining high thermal stability thereof. In order to strike the balance, CaO needs to be contained in the glass composition. On the other hand, an excessively high content of CaO causes devitrification of glass. Therefore, such an excessively high content of CaO is not preferable. From these points of view, the CaO content is 0% or more, and preferably 0.2% or more, focusing on its lower limit. The CaO content is 7% or less, and preferably 4.5% or less, focusing on its upper limit. The content of CaO is 0% or more and 7% or less, and preferably 0.2% or more and 4.5% or less.
SrO is a component for decreasing the viscosity of glass and promoting the melting and refining of the glass. The glass composition of the present invention does not necessarily need to contain SrO, but preferably it contains SrO to improve the meltability of the glass and stabilize the production of a glass substrate by the downdraw process. Furthermore, in order to strike the balance between the CaO content and the SrO content, SrO needs to be contained in the glass composition. Moreover, the glass composition of the present invention is substantially free of BaO. From this point of view, too, SrO preferably is added. On the other hand, an excessively high content of SrO decreases the acid resistance of the glass, and as a result, the glass cannot withstand the acid treatment step in the production of FPDs, for example. From these points of view, the SrO content is 0% or more, and preferably 5% or more, focusing on its lower limit. The SrO content is 13.5% or less, preferably 12% or less, and more preferably 11.5% or less, focusing on its upper limit. The content of SrO is 0% or more and 13.5% or less, preferably 0% or more and 12% or less, and more preferably 5% or more and 11.5% or less.
The glass composition of the present invention is substantially free of BaO. Therefore, the glass composition of the present invention has a low environmental load and its production cost is low.
In the present description, “substantially free” means that a trace amount of impurities that have been inevitably mixed during the industrial production of the glass composition, such as impurities derived from the raw materials, may be contained. Specifically, “substantially free” is defined as the content of less than 0.5%, preferably less than 0.3%, and more preferably less than 0.1%.
An alkaline earth metal oxide RO (where R is Mg, Ca, or Sr) is a component having an effect on the melt viscosity of glass. When the total content of ROs (MgO+CaO+SrO) is less than 10%, the meltability of the glass decreases, which makes it difficult to form a glass substrate by the downdraw process. On the other hand, when the total content thereof is more than 18.5%, the acid resistance of the glass decreases, and the glass cannot withstand the acid treatment step in the production of FDPs, for example. Therefore, the total content of ROs is 10% or more, and preferably 12% or more, focusing on its lower limit. The total content of ROs is 18.5% or less, and preferably 16% or less, focusing on its upper limit. The total content of ROs is 10% or more and 18.5% or less, preferably 10% or more and 16% or less, and more preferably 12% or more and 16% or less.
In the glass composition of the present invention, it is preferable that the mass ratio Y1/X of the MgO content Y1to the total content X of ROs be 0.2 to 0.3, the mass ratio Y2/X of the CaO content Y2to the total content X be 0.01 to 0.3, and the mass ratio Y3/X of the SrO content Y3to the total content X be 0.4 to 0.74. In this case, a glass composition having a lower devitrification temperature while maintaining high thermal stability is obtained.
Specifically, a balance is kept between the percentage of CaO in all the ROs and the percentage of SrO therein while the percentage of MgO in all the ROs is maintained in a fixed range. As a result, a glass composition having a lower devitrification temperature while maintaining high thermal stability is obtained. FIG. 1 and FIG. 2 show the dependence of the devitrification temperature of the glass composition of the present invention on the balance between the percentage of CaO and the percentage of SrO in all the ROs. In this dependence, the percentage of MgO in all the ROs is almost constant. The horizontal axis of FIG. 1 indicates the mass ratio Y2/X of the CaO content Y2to the total content X of ROs (that is, the percentage CaO/ROs of CaO in all the ROs). The horizontal axis of FIG. 2 indicates the mass ratio Y3/X of the SrO content Y3to the total content X of ROs (that is, the percentage SrO/ROs of SrO in all the ROs). Specific glass compositions are shown in Table 1 below.
As shown in FIG. 1 and FIG. 2, the devitrification temperature of the glass is lowered when the mass ratio Y1/X of MgO to ROs is 0.2 to 0.3, the mass ratio Y2/X of CaO to ROs is 0.01 to 0.3, and the mass ratio Y3/X of SrO to ROs is 0.4 to 0.74. The devitrification temperature of the glass is lowered further when the mass ratio Y1/X of MgO to ROs is 0.2 to 0.3 and the mass ratio Y2/X of CaO to ROs is 0.05 to 0.23. Likewise, the devitrification temperature of the glass is lowered further when the mass ratio Y1/X of MgO to ROs is 0.2 to 0.3 and the mass ratio Y3/X of SrO to ROs is 0.48 to 0.70. Furthermore, the devitrification temperature of the glass shows a relative minimum value in the above ranges. In the glass compositions shown in Table 1, particularly low devitrification temperatures are obtained when the mass ratio Y2/X is 0.07 to 0.13 and/or the mass ratio Y3/X is 0.60 to 0.68, although the devitrification temperature also depends on the contents of the other components.
The glass composition of the present invention may contain K2O to adjust the thermal properties of the glass. The content of K2O is 0% or more and 1% or less.
The present inventors have found that there are special relationships between the K2O content, and the devitrification temperature, the glass transition temperature (Tg) and the linear thermal expansion coefficient of the glass composition of the present invention. FIG. 3 to FIG. 5 show the dependence of the devitrification temperature, that of the Tg, and that of the linear thermal expansion coefficient of the glass composition of the present invention, on the K2O content. In these dependences, the contents of the components other than K2O are almost constant. Specific glass compositions are shown in Table 2 below.
As shown in FIG. 3, in the glass composition of the present invention, the addition of 0.1% or more of K2O to the composition brings about a tendency for the devitrification temperature to drop sharply. Meanwhile, when the K2O content exceeds 1%, the decreasing tendency of the devitrification temperature is not seen. From this point of view, the content of K2O preferably is 0.1% or more and 1% or less. A low devitrification temperature is required in order to produce a glass substrate by the downdraw process.
On the other hand, it is desirable that a glass substrate for an FPD have stable physical properties, that is, high thermal stability, even during the formation of a TFT circuit thereon. As the Tg increases and the linear thermal expansion coefficient decreases, the thermal stability of the glass is enhanced. FIG. 4 shows that when the content of K2O is 0.5% or more, the Tg drops sharply. FIG. 5 shows that when the content of K2O is more than 0.5%, the linear thermal expansion coefficient rises sharply. That is, more preferably, the content of K2O is 0.1% or more and less than 0.5%. In this case, a better balance among the devitrification temperature, Tg, and linear thermal expansion coefficient is achieved.
There is a correlation between the glass transition temperature Tg and the above-mentioned strain point, and the approximate value of the strain point can be calculated from the Tg value. Normally, the higher the glass transition temperature Tg is, the higher the strain point is.
SnO2is a Component Having an Effect of Causing Molten Glass at High temperatures to produce gas by the valence change of Sn. Such a component can be used for refining glass. Conventionally, As2O3, Sb2O3, etc. are used for refining glass. With a recent growing awareness of environmental issues, there has been a demand for glass compositions not containing these materials as refining agents. SnO2has a low environmental load and a high refining effect. Therefore, preferably, the glass composition of the present invention contains SnO2. SnO2is, however, a component that causes glass to devitrify easily, and therefore, care should be taken to prevent the content of SnO2from increasing excessively. From these points of view, the SnO2content is 0% or more, and preferably 0.1% or more, focusing on its lower limit. The SnO2content is 1% or less, and preferably 0.5% or less, focusing on its upper limit. The content of SnO2is 0% or more and 1% or less, preferably 0.1% or more and 1% or less, and more preferably 0.1% or more and 0.5% or less.
Fe2O3is a component having effects of refining and coloring glass. In Order to use the glass composition of the present invention as a glass substrate for an FPD, it is desired that the content of Fe2O3be in the range where the refining effect is enhanced but the quality of the FPD glass substrate does not deteriorate. From this point of view, the Fe2O3content is 0% or more, and preferably 0.05% or more, focusing on its lower limit. The Fe2O3content is 0.2% or less, preferably 0.15% or less, and more preferably 0.1% or less, focusing on its upper limit. The content of Fe2O3is 0% or more and 0.2% or less, preferably 0.05% or more and 0.2% or less, more preferably 0.05% or more and 0.15% or less, and further preferably 0.05% or more and 0.1% or less.
The glass composition of the present invention may contain other components, such as Li2O, Na2O, TiO2, Cl, SO3, and ZnO, as refining agents or as components for adjusting the physical properties, in addition to the above-mentioned components, as long as the total content of the other components is in the range of 0.5% or less.
The glass composition of the present invention may consist essentially of the above-mentioned components, specifically, SiO2, B2O3, Al2O3, MgO, CaO, SrO, K2O, SnO2, Fe2O3, other components added for the purpose of adjusting the physical properties, and refining agents. In this case, the glass composition of the present invention is substantially free of components other than the above-mentioned components. In this description, “consists essentially of a set of components X” means that a glass composition referred to is substantially free of components other than the set of components X.
Specifically, the glass composition of the present invention may be a glass composition consisting essentially of, in terms of mass %; 54 to 62% of SiO2; 4 to 11% of B2O3; 15 to 20% of Al2O3; 2 to 5% of MgO; 0 to 7% of CaO; 0 to 13.5% of SrO; 0 to 1% of K2O; 0 to 1% of SnO2; and 0 to 0.2% of Fe2O3. In this glass composition, the total content of alkaline earth metal oxides (MgO+CaO+SrO) is 10 to 18.5%, and the devitrification temperature is 1200° C. or lower.
Preferably, the glass composition of the present invention is a glass composition in which the content of SiO2is 55.5% or more and 60% or less, the content of B2O3is 7% or more and 11% or less, the content of Al2O3is 16% or more and 20% or less, and the content of SrO is 0% or more and 12% or less. In this glass composition, the total content of the alkaline earth metal oxides (MgO+CaO+SrO) is 10% or more and 16% or less, and the devitrification temperature is 1160° C. or lower. This also applies to the case where the glass composition of the present invention is a glass composition consisting essentially of the above-mentioned components.
Preferably, the glass composition of the present invention is a glass composition in which the content of SiO2is 56.5% or more and less than 58.4%, the content of B2O3is 8% or more and 10% or less, the content of Al2O3is 18% or more and 20% or less, the content of MgO is 3% or more and 5% or less, the content of CaO is 0.2% or more and 4.5% or less, the content of SrO is 5% or more and 11.5% or less, and the content of K2O is 0.1% or more and 1% or less. In this glass composition, the total content of the alkaline earth metal oxides (MgO+CaO+SrO) is 12% or more and 16% or less, the mass ratio Y1/X of the MgO content Y1to the total content X of the alkaline earth metal oxides ROs is 0.2 to 0.3, the mass ratio Y2/X of the CaO content Y2to the total content X of ROs is 0.01 to 0.3, and the mass ratio Y3/X of the SrO content Y3to the total content X of ROs is 0.4 to 0.74, and the devitrification temperature is 1130° C. or lower. This also applies to the case where the glass composition of the present invention is a glass composition consisting essentially of the above-mentioned components.
The devitrification temperature of the glass composition of the present invention is 1200° C. or lower, and it is decreased to 1160° C. or lower or further to 1130° C. or lower in certain compositions. The devitrification temperature can be decreased to 1120° C. or lower, 1110° C. or lower, or 1100° C. or lower in some cases, as shown in the examples described below.
The Tg of the glass composition of the present invention is 710° C. or higher, for example, and it can be raised to 720° C. or higher in certain compositions. The glass composition of the present invention having such a high Tg has high thermal stability, and therefore, it is particularly suitable for use as a glass substrate for a polysilicon LCD.
The strain point of the glass composition of the present invention is 665° C. or higher, for example, and it can be raised to 670° C. or higher in certain compositions. The glass composition of the present invention having such a high strain point has high thermal stability, and therefore, it is particularly suitable for use as a glass substrate for a polysilicon LCD.
The thermal expansion coefficient of the glass composition of the present invention is 3×10−7/° C. to 38×10−7/° C., for example, in terms of a linear thermal expansion coefficient in the range of 50° C. to 300° C. The glass composition of the present invention having such a low thermal expansion coefficient has high thermal stability, and therefore, it is particularly suitable for use as a glass substrate for a polysilicon LCD.
As described above, in the downdraw process, molten glass is allowed to flow downward along the outer wall of a glass sheet forming apparatus. Therefore, the molten glass must have a viscosity that allows the glass to flow sufficiently. Specifically, if the viscosity of the molten glass is high during the forming step, its poor fluidity causes the glass to be more susceptible to forming defects.
Generally, in the forming step of the float process, the viscosity of the molten glass fed into a float bath is about 1000 Pa·s. In contrast, in the forming step of the downdraw process, the viscosity of the molten glass must be controlled at 4000 Pa·s to 50000 Pa·s.
In addition, in the downdraw process, the creep deformation of the forming member used in the glass sheet forming apparatus causes a problem. Therefore, preferably, the glass is formed at a lower temperature. For example, it is desired that the temperature of the molten glass be 1200° C. or lower when it is in contact with the forming member. Furthermore, in the downdraw process, if the temperature of a portion of the forming member that is in contact with the molten glass (for example, the above-mentioned outer wall) is equal to or lower than the devitrification temperature of the glass, a devitrified substance is produced at a portion of the glass that is in contact with the forming member. As a result, the forming quality deteriorates, or the forming operation itself cannot be performed. For these reasons, a glass to be produced by the downdraw process is required to have a particularly low devitrification temperature, compared to a glass to be produced by the float process.
The glass composition of the present invention has a particularly low devitrification temperature, and therefore is suitable for the downdraw process. Glass forming processes other than the downdraw process can be used as long as a desired glass article can be obtained.
The glass composition of the present invention is suitable for the production of glass substrates by the downdraw process, and it is suitable for the production of glass substrates for FPDs.
The glass substrate for an FPD of the present invention is composed of the glass composition of the present invention, and has physical properties corresponding to the physical properties of the glass composition of the present invention (for example, thermal properties such as strain point, Tg, and thermal expansion coefficient).
FIG. 6 shows an FPD glass substrate of the present invention. An FPD glass substrate 51 shown in FIG. 6 is composed of the glass composition of the present invention.
The FPD glass substrate of the present invention is suitable as a glass substrate for an LCD, in particular, as a glass substrate used for a polysilicon (p-Si) LCD on which a TFT circuit is formed at a temperature ranging from 500 to 600° C.
Typically, the FPD glass substrate of the present invention is formed by forming the glass composition of the present invention by the downdraw process. That is, the FPD glass substrate of the present invention is a glass substrate obtained by forming the glass composition of the present invention by the downdraw process. In the case where the FPD glass substrate of the present invention is a glass substrate obtained by the downdraw process, it has higher surface smoothness than glass substrates obtained by other processes, and therefore is particularly suitable as a glass substrate for an LCD.
The FPDs to which the FPD glass substrate of the present invention can be applied are not limited to any particular type. The FPDs are, for example, liquid crystal displays (LCDs), electroluminescent displays (ELDs), and field emission displays (FEDs). The FPDs to which the FPD glass substrate of the present invention can be applied are typically LCDs, and particularly preferably polysilicon LCDs.
The FPD of the present invention includes the FPD glass substrate of the present invention. FIG. 7 shows an example of the FPD of the present invention. An FPD 1 shown in FIG. 7 is a polysilicon active matrix LCD. The FPD 1 includes a pair of glass substrates 2 aand 2 b, and a pair of oriented films 3 aand 3 b. The oriented film 3 ais disposed in contact with the glass substrate 2 a, and the oriented film 3 bis disposed in contact with the glass substrate 2 b. Scanning lines 4, signal lines 5, and cells 6 each having a thin film transistor (TFT) are arranged on the glass substrate 2 b. Each of the cells 6 is formed in an intersection region that is surrounded by the scanning lines 4 and the signal lines 5 in plan view. In the FPD1, at least one substrate selected from the glass substrates 2 aand 2 bis the FPD glass substrate of the present invention. In other words, this at least one of the substrates is composed of the glass composition of the present invention. Preferably, the glass substrate 2 bon which the cells 6 each having a TFT are formed, among the glass substrates 2 aand 2 b, is the FPD glass substrate of the present invention.
The type and structure of the FPD of the present invention is not limited to those of the example shown in FIG. 7, as long as the FPD includes the FPD glass substrate of the present invention.
In the production method of the present invention, the melt of the glass composition of the present invention is formed into a glass substrate for an FPD by a downdraw process. An example of the production method of the present invention is described below with reference to FIG. 8.
First, glass raw materials are melted in a melting chamber 11 to form a melt of the glass composition of the present invention. The melt formed in the melting chamber 11 is delivered to a refining chamber 12 through a pipe 13 a, and refined in the refining chamber 12. The melt thus refined in the refining chamber 12 is delivered to a downdraw forming apparatus 14 through a pipe 13 b. The melt thus delivered to the forming apparatus 14 is allowed to flow over the upper edges of the apparatus 14 and flow downward along the wall surfaces (the front wall surface and the back wall surface in FIG. 7) of the apparatus 14. These wall surfaces meet at the lower end of the apparatus 14, and two streams of the melt flowing downward along the wall surfaces are fused together at the lower end of the apparatus 14. Thus, a single glass ribbon 15 is formed. The glass ribbon 15 thus obtained is cooled, and then cut into pieces of a desired size. Thus, glass substrates for FPDs are obtained.
The details of the production method of the present invention is not limited to any particular ones as long as the melt of the glass composition of the present invention is formed into a glass substrate for an FPD by the downdraw process. The structure of the apparatus used for the production method of the present invention, that of the downdraw forming apparatus in particular, also is not limited to any particular one. EXAMPLES
First, glass raw material batches were prepared so that glass compositions of Examples and Comparative Examples shown in Tables 3 to 7 were obtained from the batches. As the glass raw materials, silica, boracic anhydride, alumina, basic magnesium carbonate, calcium carbonate, strontium carbonate, potassium carbonate, tin oxide, and iron oxide were used.
Next, each of the raw material batches thus prepared was put into a platinum crucible and allowed to stand for 2 hours in an electric furnace at a setting temperature of 1550° C., and then allowed to stand for another 2 hours in the electric furnace at a setting temperature of 1620° C. Thus, molten glass was obtained. Next, the molten glass thus obtained was poured on an iron plate to obtain a glass block. The glass block was placed in an electric furnace set at 750° C., and allowed to stand for 30 minutes, followed by cooling to 550° C. for 2 hours. Then, the electric furnace, in which the glass block was placed, was turned off so that the glass block was cooled to room temperature. Thus, a glass sample was obtained.
The devitrification temperature of the glass sample was measured in the following manner. First, the glass sample was ground in a mortar to obtain glass particles. The glass particles were subjected to sieving, and particles that passed through a sieve with a 2380 μm mesh size but did not pass through a sieve with a 1000 μm mesh size were gathered. Next, the gathered glass particles were cleaned ultrasonically in ethanol, and dried. Thus, a measurement sample was obtained. Next, 25 g of the measurement sample thus obtained was placed in a platinum boat of 12 mm in width and 200 mm in length. The platinum boat was placed in a gradient heating furnace and allowed to stand for 24 hours. Next, the platinum boat containing the glass was taken out of the furnace and cooled to room temperature. Then, crystals formed in the glass (devitrification) were observed with an optical microscope. This experiment was carried out in various ranges of measurement temperatures of the furnace, and the highest temperature at which crystals were observed was defined as the devitrification temperature of the glass sample.
The linear thermal expansion coefficient of the glass sample was measured in the following manner. First, the glass sample was worked into a cylindrical shape of 5 mm in diameter and 20 mm in length. Next, the cylindrical glass sample thus formed was subjected to thermomechanical analysis (TMA) using a thermomechanical analyzer (Thermo Plus II TMA8310, manufactured by Rigaku Corporation). In this TMA, the increase in the expansion of the glass sample was measured based on the size of the sample at a reference temperature of 50° C., and the linear thermal expansion coefficient thereof was calculated from the measured increase in the expansion. The TMA was performed at a heating rate of 5° C./min.
The Tg of the glass sample was obtained based on a TMA curve obtained to evaluate the linear thermal expansion coefficient. In this TMA curve, the temperature was plotted on the horizontal axis and the increase in the expansion of the glass sample was plotted on the vertical axis. Specifically, the Tg was obtained in the following manner. First, the TMA curve was observed from the low-temperature side to the high-temperature side. An inflection point on the TMA curve between a point where the constant rate of expansion turned into an increasing rate and a yield point where the glass sample began to contract was obtained based on the differential curve of the TMA curve. Next, two tangents were drawn at the inflection point on the TMA curve and at the point of a temperature lower by 200° C. than the temperature at the inflection point on the TMA curve. Then, the temperature at the point of intersection of these two tangents was defined as the Tg of the glass sample.
The glass composition of the present invention is suitable for a glass substrate for an FPD such as an LCD. The glass composition of the present invention is suitable as a glass substrate for an LCD, in particular, as a glass substrate used for a polysilicon (p-Si) LCD on which a TFT circuit is formed at a temperature ranging from 500 to 600° C.

Today’s LCD displays are thinner and more immersive than ever. LCD technologyis continuously evolving to improve the touch response and quality of the display. Most displays are made of glass which is extremely thin in shape typically about 0.3-0.7 mm. In LCDs, a glass substrate is used to build the TFT (Thin-film transistors) layer that controls a color filter layer and liquid crystals.
For production efficiency, manufacturers are using large-sized substrates to cut into the appropriate size requirements of the product for instance LCD display panels are produced on top of a large substrate. The glass substrates that are the foundation of panels are called ‘Mother Glass’ and the generation is determined based on the size of the mother glass.
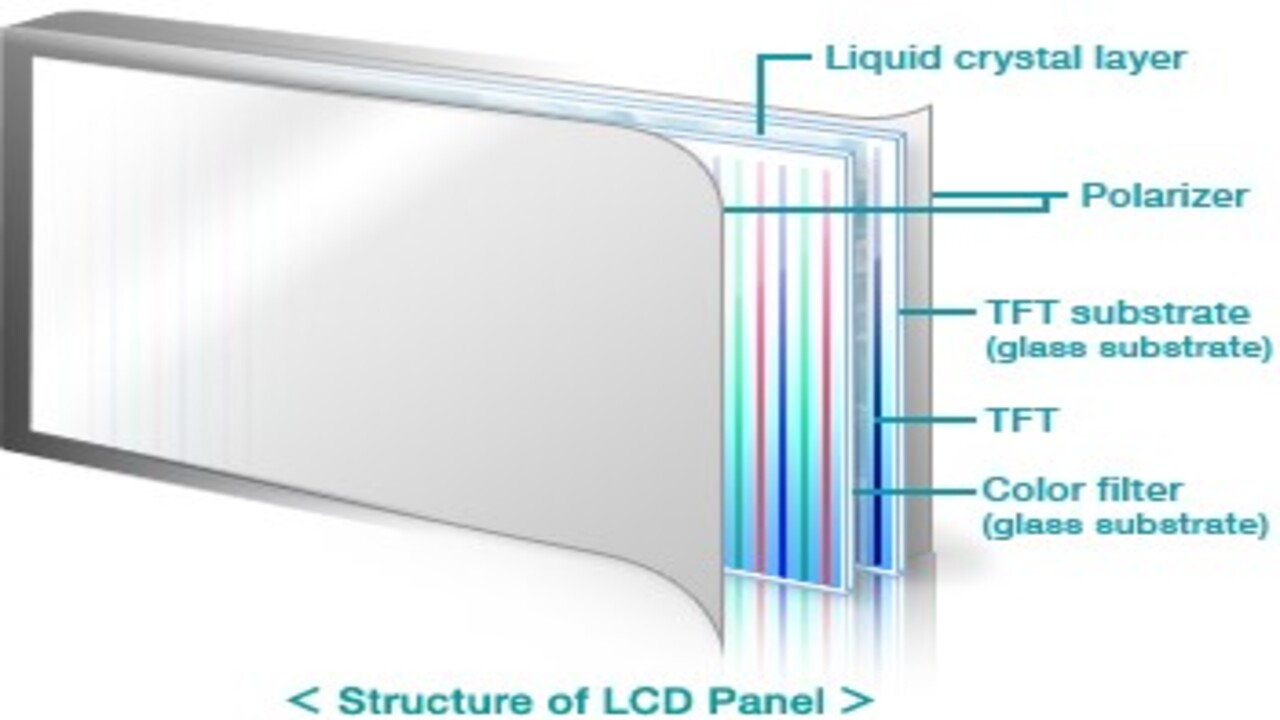
LCD glass substrate is a specialized glass used in thin-film transistors (TFTs) LCDs to form the display of various products such as personal computers, laptops, mobile phones, game consoles, automotive navigation systems, and digital cameras/camcorders. LCD glass substrate layers consist of liquid crystals, a color filter, a polarizer, TFT, and a TFT substrate.
The demand for high-resolution televisions is rapidly increasing as LCD technology is continuously growing. LCD glass substrates are widely used in televisions due to the usual size of televisions. Nowadays consumers are preferring bigger and wider televisions to enjoy vivid and fine pictures even in very large screen sizes.
TVs are usually bigger and wider compared to computers and laptops which results in increasing demand for LCD glass substrates for high-definition (HD) visuals and images.
New technological advancements in displays with higher resolution such as 4K and 8K TVs are being developed one after another which can create new pathways for the LCD glass substrates.
LCD Glass substrate enables high-resolution and rich color contrast for a better experience. The rapid growth in the television industry with large-sized screens and ultra-high definition (UHD) is likely to propel the demand for LCD glass substrates.
With this rapid growth in TV and laptop production, the globalLCD glass substrates market size was valued at USD 7879.5 million in 2020 and is anticipated to reach USD 10090 million by 2028, expanding at a CAGR of 4.2% during the forecast period, 2021–2028.
Corning Incorporated is a US-based multinational technology company that specializes in ceramics, specialty glass, and related materials and technologies including advanced optics, primarily for industrial and scientific applications.
This company has several products such as EAGLE XG Slim Glass which helps panel manufacturers to innovate for lighter, thinner, and more environmentally conscious display panels. These substrates offer smooth, clean, and flat surfaces.
Corning Astra Glass highly engineered glass substrate that enables high pixel density for high-performance displays for brighter, faster, and more realistic images. This glass offers an optimum blend of low total pitch variation (TPV), low total thickness variation (TTV), and low sag for high-performance panels.
Lotus NXT Glass is a premium glass solution designed to withstand high-temperature processing requirements with brighter, energy-efficient displays with higher resolutions while providing industry-leading levels of low total pitch variation.
Nippon Electric Glass Co., Ltd, is a Japanese glass manufacturing company. This company has several glass products based on the business such as display, consumer glass, glass fiber, electronic products, and thin film coatings.
OA-10G (Alkali-free Glass Substrate): It has excellent surface smoother because it is made by overflow technology and is widely used as a substrate for liquid crystal display and OLED display.
OA-11(Alkali-free Glass Substrate): OA-11 has particularly low deformation and deflection of gravity properties. This is both very thin and easy to handle and it is widely for OLED display and liquid crystal display, as well as a substrate for the formation of various thin films.
OA-31(High Heat-resistant and Low Thermal Compaction Glass Substrate): This is widely used in tablets and smartphones. It is suitable for OLED and LTPS displays.
LCD glass substrates are widely used in television, laptops, and automotive navigation system. Rising emerging economies and increasing technological & entertainment expenditures can produce new growth opportunities for the key players. Inventions such as the production of LCD screens using a single substrate and new upcoming substrate generations can drive market growth.

Influence of the slight adjustment of oxides on the structural and physico-chemical properties of thin film transistor-liquid crystal display substrate glass
Data from: Influence of the slight adjustment of oxides on the structural and physico-chemical properties of thin film transistor-liquid crystal display substrate glass
By the slight adjustment of oxides constituting thin film transistor-liquid crystal display (TFT-LCD) substrate glass, including equal mole fraction substitution of Al2O3, GeO2, B2O3, P2O5 and ZrO2 for SiO2, as well as the substitution of CaO for SrO with the total contents unchanged, the structural and physico-chemical properties of the glass was investigated by Raman spectroscopy and other measurements. The results showed that the short-range disorder brought by the substitution of GeO2, B2O3 and P2O5 for SiO2 could weaken the stability and compactness of the glass network, and the physico-chemical properties deteriorated, while the process of glass melting would become easier accordingly. The short-range disorder by the substitution of ZrO2 for SiO2 with 1% mole fraction showed a little difference with other samples. Finally, the substitution of modified cations, such as CaO and SrO, showed a smaller variation compared with the substitution of network formers. On the condition of 1% mole fraction substitution of oxides investigated, the variation of samples showed a reasonable change and the performance was basically all satisfied for the use of TFT-LCD substrate.
Thin film transistor-liquid crystal display (TFT-LCD) substrate glass is a kind of substrate for electronic display [1,2]. In order to meet the demand of the process of LCD devices, the glass needs to be alkali-free. The international representative products such as Corning Eagle XG™ and Asahi AN100™ mainly contain some conventional oxides, such as SiO2, Al2O3, B2O3, MgO, CaO and SrO [3,4]. The influence of some unconventional oxides on the structural and physico-chemical properties of alkali-free glass deserve further study for the improvement of the glass industry. For example, some glass network formers, such as GeO2 and P2O5, and the modified cations ZrO2, the substitution of them for SiO2 could play an important role in shaping the glass network and improving the performance of glass. Herein, we studied the influence of the slight adjustment of oxides mentioned above. To investigate the structural and physico-chemical properties of the glass, Raman spectroscopy, as well as some physico-chemical measurements were used to evaluate the effects of the slight adjustment of oxides.
Seven kinds of the chemical compositions of alkali-free glass for TFT-LCD substrate are summarized in table 1, wherein sample 1 is the reference sample for the other six samples, which are acquired by the slight adjustment of oxides from sample 1. The details are as follows: sample 2 is acquired by 1% mole fraction substitution of Al2O3 for SiO2 with equal total amount compared with sample 1, and the other samples are acquired in the same way as sample 2, including 1% mole fraction substitution of GeO2 for SiO2 for sample 3, 1% mole fraction substitution of B2O3 for SiO2 for sample 4, 1% mole fraction substitution of P2O5 for SiO2 for sample 5, 1% mole fraction substitution of SrO and CaO for sample 6 and 1% mole fraction substitution of ZrO2 for SiO2 for sample 7. By the slight adjustment of the oxides, the structural along with the physico-chemical properties were investigated.
According to the chemical compositions shown in table 1, the weighted and evenly mixed glass batch was heated in a platinum crucible in a Silicon Molybdenum Furnace with the following melting and annealing procedure: (i) heating from room temperature up to 1000°C with a heating rate of 4°C min−1; (ii) heating from 1000°C up to 1640°C with a heating rate of 2°C min−1; (iii) dwelling in 1640°C for 2 h for fining of glass liquid; (iv) pouring the glass liquid onto a copper plate for glass formation; and (v) annealing at 650°C for 1 h in a muffle furnace and then cooling down to room temperature. The prepared glass samples were cut and ground for the following measurements.
The effects of the slight adjustment of oxides on the structure of the glass was studied by Raman spectroscopy. According to the theory of irregular network of glass, the average non-bridging oxygens Onb in oxygen polyhedron is expressed by X, the average bridging oxygens Ob is expressed by Y, and Z represents the average coordination number of network formers [5–7]. As a result of RO/Al2O3 > 1, Al2O3 will be treated as network former. So the network formers are as follows: SiO2, GeO2, Al2O3, B2O3 and P2O5, while MgO, CaO, SrO and ZrO2 are treated as modified cations. Al3+ shows the priority to be coordinated by oxygen ions to form an [AlO4] tetrahedron compared with B3+ [8]. As shown in table 1, RO/Al2O3 > 1 and (RO-Al2O3)/B2O3 < 1, so Al3+ will mainly exist in the form of an [AlO4] tetrahedron, and B3+ will enter the network with more [BO3] triangle and less [BO4] tetrahedron. Table 2 shows the average coordination number Z of seven samples, wherein R represents the ratio of oxygen ions and network former ions, X = 2R − Z and Y = 2Z − 2R [5–7].
As a result of the similar components for all samples, the architecture of the glass network basically did not show too many differences, which are mainly dominated by the closer network formers such as SiO2, B2O3 and Al2O3, as well as the same total amount of modified cations of CaO, MgO and SrO. As shown in figure 1, Raman peaks for all seven samples were mainly located at three frequencies with the closer intensity, indicating that the entity and compactness of the glass network changed a little with the substitution of oxides for SiO2, proved by the closer Y and X values shown in table 2. The highest peak intensity was distributed in low frequency at around 480 cm−1 assigning to the bending vibration of Si–Ob in Si–Ob–Si bonds [9]. The second highest peak intensity was in the region of 850–1250 cm−1 relating to the stretching vibration of bridging oxygens Si–Ob and the stretching vibration of non-bridging oxygens Si–Onb [10,11]. The third highest peak intensity was in the middle frequency at around 803 cm−1 relating to the symmetric stretching vibration of Si–Ob–Si between [SiO4] tetrahedrons [9,12]. Some weaker intensity peaks distributed at around 710 cm−1, 1310 cm−1 and 1440 cm−1 could attribute to the vibration of the B–O bond, wherein a peak at 710 cm−1 is assigned to B–O–B bending vibration in the [BO3] triangle [13], and peaks located at around 1310 cm−1 and around 1440 cm−1 are assigned to B–Ob stretching vibration and B–Onb stretching vibration in the [BO3] triangle, respectively [14].
For samples 1–7, there were three main fit peaks in this region. The first peak with the highest intensity was at a frequency of around 1030 cm−1, corresponding to stretching vibration of bridging oxygens Si–Ob. Some researchers assumed that this peak should be owing to the vibration of bridging oxygens except Q4 unit [16,17]. As Q1 and Q2 units were not obviously found in this study, the peak located at around 1030 cm−1 should be derived from the Q3 unit. The second highest peak intensity located at around 1170 cm−1 corresponded to stretching vibration of bridging oxygens Si–Ob in the Q4 unit [16,17]. The weakest peak was located at around 1110 cm−1, corresponding to stretching vibration of non-bridging oxygens Si–Onb in the Q3 unit. As shown in figure 2a, peaks at around 1030 cm−1 and 1170 cm−1 accounted for most of the region, indicating that the vibration of bridging oxygens Si–Ob dominates in alkali-free glasses, in accordance with the higher Y value roughly estimated in table 2.
Secondly, compared with reference sample 1, samples 2 and 4 show a higher ratio of Al/Si. With the replacement of Si4+ by Al3+, some non-bridging oxygens will coordinate Al3+ to form an [AlO4] tetrahedron to connect with the [SiO4] tetrahedron in the glass network, and these non-bridging oxygens are the very ones from the Q3 structure unit. So with the increase of Al/Si, the Q3 unit decreased and the Q4 unit increased. In the same way, for samples 3 and 5, some Si4+ were replaced by Ge4+ or P5+, accordingly. The Q3 unit decreased with the decrease of SiO2 and can be connected with the [GeO4] or [PO4] tetrahedron to form the Q4 unit. So the strengthening of Q4 vibration probably came from Si–Ob–Al caused by the formation of an [AlO4] tetrahedron for samples 2 and 4, and Si–Ob–Ge, Si–Ob–P for samples 3 and 5. This is why the Si–Onb vibration at around 1110 cm−1 weakened, while the Si–Ob vibration at 1170 cm−1 strengthened for samples 2, 3, 4 and 5. Basically, with the higher ratio of Al/Si, Si–Ob–Si and Si–Ob–B decreased, while Si–Ob–Al increased, along with the newcomer Si–Ob–Ge or Si–Ob–P, bringing more distortion into the glass network compared with sample 1, causing the increase of the disorder degree of the network and lower frequency shift of the peaks.
It can be derived and inferred from above, the low frequency bending vibration of Si–Ob in Si–Ob–Si bonds dominated in alkali-free glass, and B3+ was mainly found in the form of a [BO3] triangle owing to its inferiority to Al3+ coordinated by oxygen ions and fewer alkali-earth ions. As for the [SiO4] tetrahedron, Q3 and Q4 units accounted for the most in four kinds of [SiO4] units. With the substitution of oxides for SiO2, Q3 decreased and Q4 increased, but the disorder degree of the network increased, embodied in the lower frequency shift of the Raman peak. Sample 7 with Zr4+ showed more peak shift than others, mainly owing to the high electric field of Zr4+ that can significantly affect the network of glass.
The thermal expansion softening temperature were between 767°C and 791°C for seven samples. They also showed a good agreement with the tightness of the glass network as discussed above.
Table 5 lists the optical properties of the seven samples, including the average transmittance in visible light and the refractive index. All seven samples were cut and ground to the same thickness of 2 mm. As shown in table 5, the average transmittance of seven samples were very close to each other with the values of 90–91%, as well as the refractive index at around 1.520–1.526, showing very small differences with each other. From the analysis of the Raman spectra above, the glass network of all samples with the slight adjustment of oxides did not show too much difference. So the optical properties which were mainly dominated by the network and compositions exhibited similar performances accordingly.
Meanwhile, sample 7 showed a little smaller transmittance and a little larger refractive index than other samples. The extranuclear electrons of Zr4+ were easily polarized owing to the larger radius of Zr4+, and the refractive index of glass relates to the polarity of electrons. The increasing refractive index also affects the transmittance [22]. As a result of small amount of substitution of ZrO2 for SiO2 for sample 7, the effect of Zr4+ was not too significant.
Table 6 shows the logarithm volume resistivity of samples measured at 25°C and 250°C, respectively. TFT-LCD substrate glass is a kind of alkali-free glass and the alkali-earth ions are the primary conductive elements, which show a lower conductivity than alkaline ions. This is why the volume resistivity of TFT-LCD substrate glass is larger than conventional soda lime glass. At a temperature of 25°C, the logarithm volume resistivities of the TFT-LCD substrate glass is between 15.1 Ω cm and 15.9 Ω cm. When the samples were heated, the energy of alkali-earth ions increased, and the number of ions with high enough energy in the glass network that could migrate in the electric field also increased, causing the decrease of the resistivity to around 13 Ω cm at 250°C shown in table 5, which declined about 100 times compared with that at 25°C.
The curves of high temperature resistivity for the seven samples are shown in figure 4. The resistivities decreased with the temperature ranging from 1300°C to 1600°C. When the temperature was not high enough to destroy the network of glass, for example, at 1300–1400°C, alkali-earth ions will move in the gap of the network to conduct.
As for the comparison of samples 3 and 1, the resistivity became lower by the substitution of GeO2 for SiO2. Because the volume of [GeO4] is larger than the [SiO4] tetrahedron, the network of glass expands to generate a more relaxed structure, which benefited from the movement for alkali-earth ions and reduced the resistivity for sample 3.
Finally, with the increase of temperature, the glass network vanished for all seven samples, and the mechanism of conduction was the free movement of alkali-earth ions in molten glass liquid, leading to the same value in the final molten state for about 50 Ω·cm for all seven samples whose number of alkali-earth ions were equal.
Figure 5 shows the curves of high temperature viscosity of the seven samples in the range of 1100–1700°C, wherein data ranging from 1200°C to 1550°C were the measured values, corresponding to viscosity of 101.5–103.2 Pa s, and data beyond that were fitted by the Fulcher equation [23,24]. As a matter of fact, the difference between measured data and fitted data was less than 1°C. So in figure 5, only the smooth curves of fitted data were shown. Table 7 shows some reference points of viscosity-temperature characteristics for float glass, including melting point of glass (Tm), forming point of glass (Tf), working point of glass (Tw) and the range for polishing area and slow cooling area.
Secondly, GeO2 is easier to become molten than SiO2, and the substitution of GeO2 for SiO2 will bring the short-range disorder as a result of the different volumes of [GeO4] and [SiO4] tetrahedrons in the glass network, making the network loose and the reference points of viscosity-temperature characteristics decrease. By comparison of sample 3 and sample 1, melting point decreased for 8°C and working point decreased for 14°C with the substitution of 1% mole fraction.
Sample 4 was acquired by the substitution of B2O3 for SiO2 with 1% mole fraction. As a result of (RO-Al2O3)/B2O3 < 1, with the increase of B2O3 and decrease of SiO2, the [SiO4] tetrahedron decreased and the [BO3] triangle increased, increasing the disorder and lowering the compactness of the network, and thus making the viscosity of glass liquid reduced. By comparison of sample 4 and sample 1, with the increase of B2O3 and decrease of SiO2 for 1% mole fraction, melting point and working point decreased for 11°C and 9°C, respectively.
For sample 5 and sample 1 with the substitution of P2O5 for SiO2, the reference points of viscosity-temperature characteristics decreased more than the other samples. P2O5 can lower the melting point probably owing to its small viscous activation energy, meanwhile, P5+ can form a [PO4] tetrahedron and enter the network with the P=O double bond, which is an asymmetry centre and will make the structure relaxed and thus lower the viscosity of the glass liquid. By comparison of sample 5 and sample 1, melting point and working point decreased for 18°C and 25°C, respectively, exhibiting the most decrease for all samples.
By the slight adjustment of oxides of the TFT-LCD substrate glass, including equal mole fraction substitution of Al2O3, GeO2, B2O3, P2O5 and ZrO2 for SiO2, as well as the substitution of CaO for SrO with the total contents unchanged, the structure and physico-chemical properties of glass was investigated. The results were as follows.(i) The architecture of the glass network did not show too much difference with the substitution of 1% mole fraction for network formers and modified cations. The low frequency bending vibration of Si–Ob in Si–Ob–Si bonds dominated in alkali-free glass, and B3+ was mainly found in the form of a [BO3] triangle owing to its inferiority to Al3+ coordinated by oxygen ions and fewer alkali-earth ions. As for the [SiO4] tetrahedron, Q3 and Q4 units accounted for the most in four kinds of [SiO4] units. With the substitution of oxides for SiO2, Q3 decreased and Q4 increased, but the disorder degree of network increased, embodied in the lower frequency shift of the Raman peak. Sample 7 with Zr4+ showed more peak shift than others, mainly owing to the high electric of Zr4+ that can significantly affect the glass network.
(ii) The stability and entirety of the glass network deteriorated by substitution of GeO2, B2O3, P2O5 for SiO2, and some physiochemical property indexes got worse, such as the decrease of elastic modulus and shear modulus for the mechanical properties, the increase of the thermal expansion coefficient for the thermal properties, and the decrease of the reference points of viscosity-temperature characteristics. Accordingly, the process for float glass became easier by the substitution, which presented a contrary tendency. For example, the melting point of the glass and the resistivity of glass liquid both decreased, which would contribute to the melting process of glass.
(iii) For the substitution of Al2O3 and ZrO2 for SiO2, the stability of the network did not show significant deterioration, probably owing to the [AlO4] tetrahedron and the [ZrO6] octahedron. They can enter the network to connect with the [SiO4] tetrahedron and the compactness could be strengthened and the property indexes correspondingly became better, such as the increase of elastic modulus and shear modulus for the mechanical properti




 Ms.Josey
Ms.Josey 
 Ms.Josey
Ms.Josey