tft lcd glass substrate quotation

"TFT-LCD Glass Substrate Market" report presents a comprehensive overview, market shares, and growth opportunities of TFT-LCD Glass Substrate market by product type, application, key manufacturers and key regions and countries. The global TFT-LCD Glass Substrate market size is projected to reach Multimillion USD by 2028, in comparision to 2021, at unexpected CAGR during 2022-2028.
TFT-LCD Glass SubstrateMarket Research Report is spread across 85 Pages with 130 Number of Tables and Figures that provides exclusive data, information, vital statistics, trends, and competitive landscape details in this niche sector.
The Global TFT-LCD Glass Substrate market is anticipated to rise at a considerable rate during the forecast period, between 2022 and 2028. In 2020, the market is growing at a steady rate and with the rising adoption of strategies by key players, the market is expected to rise over the projected horizon.
Due to the COVID-19 pandemic, the global TFT-LCD Glass Substrate market size is estimated to be worth USD million in 2022 and is forecast to a readjusted size of USD million by 2028 with a CAGR of % during the forecast period 2022-2028. Fully considering the economic change by this health crisis, Gen. 5 accounting for % of the TFT-LCD Glass Substrate global market in 2021, is projected to value USD million by 2028, growing at a revised % CAGR from 2022 to 2028. While Television segment is altered to an % CAGR throughout this forecast period.
North America TFT-LCD Glass Substrate market is estimated at USD million in 2021, while Europe is forecast to reach USD million by 2028. The proportion of the North America is % in 2021, while Europe percentage is %, and it is predicted that Europe share will reach % in 2028, trailing a CAGR of % through the analysis period 2022-2028. As for the Asia, the notable markets are Japan and South Korea, CAGR is % and % respectively for the next 6-year period.
The global major manufacturers of TFT-LCD Glass Substrate include Corning, AGC, NEG, Tunghsu Optoelectronic, AvanStrate, IRICO and LG Chem, etc. In terms of revenue, the global 3 largest players have a % market share of TFT-LCD Glass Substrate in 2021.
Report further studies the market development status and future TFT-LCD Glass Substrate Market trend across the world. Also, it splits TFT-LCD Glass Substrate market Segmentation by Type and by Applications to fully and deeply research and reveal market profile and prospects.
Geographically, this report is segmented into several key regions, with sales, revenue, market share and growth Rate of TFT-LCD Glass Substrate in these regions, from 2015 to 2027, covering ● North America (United States, Canada and Mexico)
Some of the key questions answered in this report: ● What is the global (North America, Europe, Asia-Pacific, South America, Middle East and Africa) sales value, production value, consumption value, import and export of TFT-LCD Glass Substrate?
● Who are the global key manufacturers of the TFT-LCD Glass Substrate Industry? How is their operating situation (capacity, production, sales, price, cost, gross, and revenue)?
● What are the upstream raw materials and manufacturing equipment of TFT-LCD Glass Substrate along with the manufacturing process of TFT-LCD Glass Substrate?
● What are the key drivers, restraints, opportunities, and challenges of the TFT-LCD Glass Substrate market, and how they are expected to impact the market?

This report presents an extensive outline, pieces of the overall industry, and development chances of Glass Substrates for TFT-LCD market by item type, application, key makers and key locales and nations.
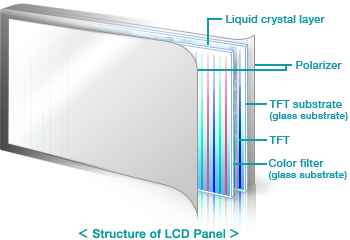
Glass Substrate is a special glass used for thin-film-transistor (TFT) liquid crystal displays (LCD) and OLEDs, which form the display area of products including LCD televisions, personal computers and mobile phones. An LCD panel consists of various components stacked in a number of layers. These components include a polarizer, a color filter and a liquid crystal layer, with the glass substrate being the most important.
According to our latest study, the global Glass Substrates for TFT-LCD market size was valued at USD 7271.6 million in 2021 and is forecast to a readjusted size of USD 11450 million by 2028 with a CAGR of 6.7% during review period. The influence of COVID-19 and the Russia-Ukraine War were considered while estimating market sizes.
This report is a detailed and comprehensive analysis for global Glass Substrates for TFT-LCD market. Both quantitative and qualitative analyses are presented by manufacturers, by region and country, by Type and by Application. As the market is constantly changing, this report explores the competition, supply and demand trends, as well as key factors that contribute to its changing demands across many markets. Company profiles and product examples of selected competitors, along with market share estimates of some of the selected leaders for the year 2022, are provided.
Additionally, the Glass Substrates for TFT-LCD market trend provides a comprehensive study of prime players at intervals the market by lightness their product description, business outline, and business strategy. It conjointly endows with the amount of production, Glass Substrates for TFT-LCD"s future demand, needed staple, and also the cash health of the organization.
The Glass Substrates for TFT-LCD Market report purposefully analyses every sub-segment regarding the individual growth trends, contribution to the total market, and the upcoming forecasts.
For the end users/applications, this report focuses on the status and outlook for major applications/end users, consumption (sales), market share and growth rate of Glass Substrates for TFT-LCD market for each application, including: -
This report studies the global market size of Glass Substrates for TFT-LCD in key regions like North America, Europe, China and Japan, focuses on the consumption of Glass Substrates for TFT-LCD in these regions.
Key questions answered in the report: ● What are the Glass Substrates for TFT-LCD market opportunities and threats faced by the vendors in the Global Glass Substrates for TFT-LCD industries?
3) Focuses on the key Glass Substrates for TFT-LCD manufacturers, to study the capacity, production, value, market share and development plans in the future.
Global Glass Substrates for TFT-LCD Industry 2023 Market Research Report provides exclusive vital statistics, data, information, trends and competitive landscape details in this niche sector.
Display glass substrate is a special glass used for supporting TFT (Thin Film Transistor), LCD (Liquid Crystal Display) and OLED panels forming display units for products including televisions, personal computers and mobile phones. Ever display panel consists of various components stacked in a number of layers, which includes a color filter, a polarizer and a liquid crystal display with the glass substrate being the most important.
In 2016, the display glass substrate market was valued at US$ 1.42 Billion and is projected to reach US$ 1.97 Billion by 2028, growing at a CAGR of 5.7%. With the expanding demand for consumer grade electronic devices such as smartphones, laptops and personal computers, the global display glass substrate market is expected to grow during the forecast period.
The expanding use of LCD’s in smart handheld devices, consumer durables, and other automotive applications is one of the most powerful factors projected to drive the growth of the display glass substrate market. Moreover, the advancements in the electronics and semiconductor industries are further projected to drive the increasing demand for display glass substrates.
The electronics industry is the largest end-using commerce segment that suitably utilizes display glass substrates and the general growth for the display glass substrate market is heavily dependent on it. Increasing in manufacturing of display devices, electronic components, semiconductor devices, MEMS (Microelectronic Mechanical System) devices, and computing & telecommunication devices is expected to drive the growth of the display glass substrate market.
However, the immense manufacturing cost of display glass substrates acts as a restraint to the expansion of the display glass substrate market. Manufacturers of display glass substrates are focusing on directing their earnings through several process control techniques with the aim of optimizing production costs to a certain level.
Asia Pacific is accredited to be the largest market for display glass substrates. The display glass substrate market in the Asia Pacific region is estimated to grow at the highest CAGR during the forecast period owing to the occupancy of numerous electronics manufacturers in this region.
China, Hong Kong and South Korea are expected to account for the largest share of the glass substrate market in the Asia Pacific region in 2028 as most of the major producers of display glass substrates such as Nippon Sheet Glass (Japan) and The Tungshu Group (China) are located in the Asia Pacific region. The Asia Pacific region accounts for over 37% of the total display glass substrate market. Other regions include North America and Europe with a market share of 8% and 3% in the global display glass substrate market.
Some of the key players in the global display glass substrate market are Corning Incorporated, LG Chem, AGC Incorporated, AvanStrate Incorporated, SCHOTT Ag, Tungshu Optoelectronics, IRICO Group New Energy Company Limited and CGC Glass.
The research report presents a comprehensive assessment of the display glass substrate market and contains thoughtful insights, facts, historical data and statistically supported and industry validated market data. It also includes projections using a suitable set of assumptions and methodologies. The research report of display glass substrate market provides analysis and information according to the different market segments such as geographies, grade type and application.
The display glass substrate market report is a compilation of first-hand information, qualitative and competitive assessment industry analysts, inputs from industry experts and industry participants across the value chain. The report for display glass substrate market provides an in depth analysis of parent market trends, macro-economic indicators and governing factors along with market attractiveness as per segments. The report also maps the qualitative impact of various market factors on market segments and geographies.

TFT liquid crystal display is characterized by good brightness, high contrast, strong sense of hierarchy, bright colors, but there are also relatively power consumption and high cost deficiencies. TFT liquid crystal technology has accelerated the development of cell phone color screen. Many of the new generation of color screen cell phones support 65536 color display, some even support 160,000 color display, this time TFT’s high contrast, color-rich advantages are very important.
TFT technology is developed in the 1990s, using new materials and processes of large-scale semiconductor fully integrated circuit manufacturing technology, is the liquid crystal (LC), inorganic and organic thin film electroluminescent (EL and OEL) flat panel display basis. TFT is in the glass or plastic substrate and other non-monocrystalline wafer (of course, can also be on the wafer) through sputtering, chemical deposition process to form the necessary manufacturing circuit TFT is formed on a non-monocrystalline substrate such as a glass or plastic substrate (or, of course, on a wafer) by sputtering and chemical deposition processes to form the various films necessary for the manufacture of circuits. The use of non-monocrystalline substrates can significantly reduce costs and is an extension of conventional large-scale integrated circuits to large-area, multi-functional, low-cost direction. It is more technically difficult to manufacture TFTs that control the switching performance of image elements (LC or OLED) on large-area glass or plastic substrates than to manufacture large-scale ICs on silicon wafers. The requirements for the production environment (purification level 100), the requirements for the purity of raw materials (99.999985% purity of electronic special gas), the requirements for production equipment and production technology exceed those of semiconductor mass integration, which is the top technology of modern mass production. Its main features are.
(1) large area: the first generation of large-area glass substrate (300mm × 400mm) TFT-LCD production line put into operation in the early nineties, to the first half of 2000 the area of the glass substrate has been expanded to 680mm × 880mm), and 950mm × 1200mm glass substrate will also be put into operation. In principle, there is no area limitation.
(2) high integration: 1.3-inch TFT chip for LCD projection resolution of XGA contains millions of pixels. Resolution of SXGA (1280 × 1024) of 16.1-inch TFT array non-crystalline silicon film thickness of only 50nm, as well as TABONGLASS and SYSTEMONGLASS technology, the integration of its IC, the requirements of equipment and supply technology, technical difficulties are more than the traditional LSI.
(3) Powerful: TFT was first used as a matrix addressing circuit to improve the light valve characteristics of liquid crystals. For high-resolution displays, precise control of the object element is achieved through voltage regulation in the range of 0-6V (its typical value of 0.2 to 4V), thus making it possible for LCDs to achieve high-quality, high-resolution displays. TFT-LCD is the first flat panel display in human history that exceeds CRT in display quality. And people began to integrate the driver IC into the glass substrate, the whole TFT will be more powerful, which is unmatched by the traditional large-scale semiconductor integrated circuits.
(4) low cost: glass substrates and plastic substrates fundamentally solve the cost of large-scale semiconductor integrated circuits, the application of large-scale semiconductor integrated circuits to open up a wide application space.
(6) wide range of applications, TFT technology-based liquid crystal flat panel display is the pillar of the information society, but also technology can be applied to the rapid growth of thin-film transistor organic electroluminescent (TFT-OLED) flat panel display is also growing rapidly.
With the maturity of TFT technology in the early nineties, color LCD flat panel displays developed rapidly, and in less than 10 years, TFT-LCD grew rapidly into a mainstream display, which is inseparable from the advantages it has. The main features are.
(1) the use of good characteristics: low-voltage applications, low drive voltage, solidification of the use of security and reliability; flat, and thin, saving a lot of raw materials and use of space; low power consumption, its power consumption is about one tenth of the CRT display, reflective TFT-LCD is even only about one percent of the CRT, saving a lot of energy; TFT-LCD products and specifications models, the TFT-LCD products also have many features such as serialized specifications, various sizes, convenient and flexible usage, easy maintenance, updating and upgrading, and long service life. Display range covers the application range of all displays from 1 inch to 40 inches and projection of large planes, is a full-size display terminal; display quality from the simplest monochrome character graphics to high resolution, high color fidelity, high brightness, high contrast ratio, high response speed of various specifications of the model video display; display mode has a direct view type, projection type, see-through type, but also reflective type.
(2) Good environmental characteristics: no radiation, no flicker, no damage to the user’s health. Especially the emergence of TFT-LCD electronic books, will bring mankind into the paperless office, paperless printing era, triggering a revolution in the way humans learn, spread and record civilization.
(3) Wide range of application, from -20 ℃ to +50 ℃ temperature range can be used normally, after temperature reinforced processing of TFT-LCD low temperature working temperature can reach minus 80 ℃. It can be used as mobile terminal display, desktop terminal display, and large screen projection TV, which is a full-size video display terminal with excellent performance.
(4) The manufacturing technology is highly automated and has good characteristics for large-scale industrial production, and the technology of TFT-LCD industry is mature and the yield rate of large-scale production is over 90%.
(5) TFT-LCD is easy to integrate and update, and is a perfect combination of large-scale semiconductor integrated circuit technology and light source technology, with great potential for continued development. Currently there are amorphous, polycrystalline and monocrystalline silicon TFT-LCD, the future will have other materials TFT, both glass substrate and plastic substrate.

Color LCD module PS302-04043-00 is composed of the amorphous silicon thin film transistor liquid crystal display (a-Si TFT LCD) panel structure with driver LSIs for driving the TFT (Thin Film Transistor) array and a dual mode backlight. The a-Si TFT LCD panel structure is injected liquid crystal material into a narrow gap between the TFT array glass substrate and a color-filter glass substrate. Color (Red, Green, Blue) data signals from a host system (e.g. signal generator, etc.) are modulated into best form for active matrix system by a signal processing board, and sent to the driver LSIs which drive the individual TFT arrays. The TFT array as an electro-optical switch regulates the amount of transmitted light from the backlight assembly, when it is controlled by data signals. Color images are created by regulating the amount of transmitted light through the TFT array of red, green and blue dots.

In the following description, the situation of carrying out the colored TFT display panels that shows with display panels is that example describes, but the present invention also can be applicable to TFT display panels display panels in addition.In addition, can also be applicable to the display panels that carries out monochrome (monochrome) demonstration.
Fig. 1 represents glass substrate paired in the display panels of the present invention.On first glass substrate 10, dispose TFT, show electrode, source electrode distribution, gate wirings etc. (diagram slightly).And on second glass substrate 20, dispose as the color material film of colored filter and common electrode etc. (diagram slightly).First glass substrate 10 and second glass substrate, 20 holding liquid crystals (diagram slightly) and relative form display panels thus.
But first glass substrate 10 is relative in the mode of mutual grain direction quadrature with second glass substrate 20.That is, relative with the mode of the grain direction quadrature of second glass substrate 20 with the grain direction of first glass substrate 10.
In other words, two relative glass substrates can be textures that has along short side direction, and another has the texture along long side direction.And, do not get rid of the identical situation of length on minor face and long limit, so long as the grain direction quadrature of the grain direction of a glass substrate and another glass substrate gets final product.
First glass substrate 10 is relative in the mode of mutual grain direction quadrature with second glass substrate 20, thereby the randomness of the deviation in the gap between the glass substrate 10,20 improves.Its result, the deviation in gap becomes not obvious, can obtain good display quality.That is, though because of the existence of texture has the little zone in big zone, gap and gap, because the grain direction quadrature, so these each zones are separated into the very little zone of area respectively and exist.If the little zone in zone that the gap is big and gap has area to a certain degree separately, show that then inequality can become obvious, but exist by making the little zone in big zone, gap and gap be separated into the very little zone of area respectively, even thereby the gap heterogeneity also can access the uneven and unconspicuous good display quality of demonstration.
Fig. 2 is the synoptic diagram of the structure example of expression display panels of the present invention.Display panels of the present invention possesses as mentioned above with the mode of mutual grain direction quadrature relative first glass substrate 10 and second glass substrate 20.
First glass substrate 10 is provided with TFT12 and show electrode 11, is formed with alignment film 13 on show electrode 11.Thereby first glass substrate 10 can be known as the TFT glass substrate.TFT12 is corresponding one by one with show electrode 11, and the location of each show electrode 11 becomes the pixel in the display panels.In addition, be connected with source electrode distribution and gate wirings (diagram slightly) on each TFT12.Show electrode 11 is according to the voltage that is supplied to TFT12 by source electrode distribution and gate wirings and Be Controlled voltage.
Second glass substrate 20 is provided with color material film 21R, 21G, 21B and the black matrix 22 as colored filter.Thereby second glass substrate 20 can be known as the colored filter glass substrate.In addition, be formed with diaphragm 23, on diaphragm 23, be formed with common electrode 24 and alignment film 25 in the mode that covers color material film 21R, 21G, 21B and black matrix 22.
Configuration to color material film 21R, 21G, 21B and show electrode 11 describes in more detail.Show electrode 11 is disposed at first substrate 10, with color material film 21R, when 21G, 21B are disposed at second substrate 20, so that the grain direction of first substrate 10 and the mode of the grain direction quadrature of second substrate 20 when making first substrate 10 relative with second substrate 20, configuration color material film 21R, 21G, 21B and show electrode 11 make that the allocation position of the allocation position of color material film 21R, 21G, 21B and each show electrode 11 is overlapping.
In the illustrated display panels of Fig. 2, first substrate 10 and second substrate 20 dispose in the mode of the grain direction quadrature of the grain direction of first substrate 10 and second substrate 20.By adopting such structure, as illustrating,, show uneven and unconspicuous good display quality so can access because the big little zone in zone and gap, gap is separated into the very little zone of area respectively and exists.
In addition,, also can access good display quality, so can be less to the amount of grinding of adopting the glass raw sheet that plate obtains from glass tape even there are texture in first substrate 10 and second substrate 20.Therefore, can shorten the time that the glass raw sheet adds the milled processed in man-hour.
Then, the manufacture method to display panels of the present invention describes.Fig. 3 is the process flow diagram of example of the manufacture method of expression display panels of the present invention.At first, adopt plate from glass tape and go out the glass raw sheet of long fringe reason of identical size and the glass raw sheet (step S1) of minor face texture.Fig. 4 is the key diagram of long fringe reason of expression and minor face texture.Long fringe reason is meant shown in Fig. 4 (a), along the texture of the length direction (that is long side direction) of glass plate.And the minor face texture is meant shown in Fig. 4 (b), along the texture of the short side direction of glass plate.
Fig. 5 is illustrated among the step S1 key diagram of example of adopting the glass raw sheet of the glass raw sheet of the long fringe reason of plate and minor face texture from glass tape.As shown in Figure 5, the glass raw sheet 41 of rectangle that can long side direction is identical with the bearing of trend of glass tape 40 is adopted plate as the glass raw sheet of long fringe reason, and the glass raw sheet 42 of the rectangle that short side direction is identical with the bearing of trend of glass tape 40 is adopted plate as the glass raw sheet of minor face texture.But Fig. 5 is the illustration of adopting the plate method among the step S1, also can utilize other method to adopt plate.For example, have following situation: adopt the glass raw sheet of the long fringe of plate reason from glass tape 40, vertically cut apart this glass raw sheet with long limit after, obtain the glass raw sheet of minor face texture.Also can be like this will adopt that glass raw sheet that plate obtains is cut apart and the glass raw sheet that cuts out the expectation texture.
In addition, the glass tape that uses in step S1 for example can use the glass tape that utilizes float glass process to generate, but also can use the glass tape that utilizes float glass process method (for example longitudinal stretching method for making) in addition to generate.
After step S1, the glass raw sheet 41 of the long fringe reason that cut out and the glass raw sheet 42 of minor face texture are implemented processing (step S2) such as chamferings and grinding.But, in milled processed, can not be ground to till the degree of texture disappearance, can on the glass plate after the processing of step S2, manage or the minor face texture by residual long fringe.
Then, on the glass plate of the glass plate of the long fringe reason that obtains by step S2 and minor face texture, form various parts (step S3).That is, with in the glass plate of the glass plate of long fringe reason and minor face texture any as first glass substrate 10 (with reference to Fig. 2), with another as second glass substrate 20 (with reference to Fig. 2).Also the either party of the glass plate of the glass plate that long fringe can be managed and minor face texture is as first glass substrate 10.Then, on first glass substrate 10 that for example rectangular configuration of the combination of TFT12 and show electrode 11 is a plurality of, and on show electrode 11, form alignment film 13.In addition, on second glass substrate 20, form color material film 21R, 21G, 21B and black matrix 22, and form diaphragm 23 on their upper strata.At this moment, make first substrate 10 and second substrate 20 with the grain direction of first substrate 10 when relative with the mode of the grain direction quadrature of second substrate 20, configuration color material film 21R, 21G, 21B and show electrode 11 make that the allocation position of the allocation position of color material film 21R, 21G, 21B and each show electrode 11 is overlapping.In addition, on the diaphragm 23 that is formed at second substrate 20, form common electrode 24, and then form alignment film 25 on described common electrode 24 upper stratas.And each alignment film 13 and alignment film 25 carried out friction treatment.
In addition, in step S3,, get with the multiaspect that can carry out display panels to the parts of the amount of first glass substrate 10 and a plurality of display panels of second glass substrate 20 configuration.
After step S3, in first substrate 10 and second substrate 20, form encapsulant 30 (with reference to Fig. 2) on any one or two, make first substrate 10 and the texture with the mode of the texture quadrature of second substrate 20 relative (step S4) of second substrate 20 with first substrate 10.Its result can access the aggregate of a plurality of dummy cells (cell) by the dummy cell of the state of glass substrate 10,20 connections.Subsequent steps S4 cuts out each display panels (being dummy cell constantly at this) (step S5).That is, the multiaspect of carrying out dummy cell is got.Then, each dummy cell is injected liquid crystal (step S6).
By above processing, obtain a plurality of display panels of the grain direction quadrature of the grain direction of first substrate 10 and second substrate 20.As previously discussed, in this display panels, can realize good display quality.In addition, the time of the milled processed among the step S2 can be shorter.
The present invention relates to a glass composition, and more particularly to a glass composition suitable for a glass substrate for a flat panel display (FPD) such as a liquid crystal display (LCD). The present invention also relates to an FPD glass substrate using this glass composition, a flat panel display, and a method of producing a glass substrate for a flat panel display.
There has been an increasing demand for flat type image display apparatuses called “flat panel displays (FPDs)” such as liquid crystal displays (LCDs). Among the FPDs, active matrix LCDs using thin film transistors (TFTs) have been widespread because they display high quality images. In an active matrix LCD, a TFT circuit is formed on the surface of a glass substrate. Conventionally, the step of forming a TFT circuit on the surface of a glass substrate is carried out in an environment of 1000° C. or higher. In recent years, however, a low-temperature polysilicon (p-Si) active matrix LCD, in which a TFT circuit can be formed at a temperature of 500 to 600° C., has been developed. This development makes it possible to use not only silica glass having stable physical properties under high temperature conditions but also aluminosilicate glass and aluminoborosilicate glass as glass substrates for LCDs.
An FPD glass substrate is required to have a small thickness and a very smooth surface. In addition, there is a strong demand for the production of larger glass substrates in response to a recent increase in the size of FPDs. There are various methods of producing glass substrates, and among them, a downdraw process is the best method for obtaining such glass substrates efficiently. In the downdraw process, molten glass is fed into a trough formed in the upper part of a glass sheet forming apparatus, and the molten glass flowing over the both edges of the trough is allowed to flow downward along the outer wall of the forming apparatus. Then, two streams of molten glass are fused together at the lower end (root) of the forming apparatus so as to produce a single glass ribbon continuously. After the glass ribbon solidifies, it is cut into pieces of a desired size. Thus, glass substrates are obtained.
Compared with a float process, which is another method for producing glass substrates, the downdraw process has a drawback in that glass substrates are susceptible to devitrification because they are formed at a lower temperature. Therefore, a glass composition having a low devitrification temperature is required in order to produce a glass substrate stably by the downdraw process. In addition, in order to form a TFT circuit on a glass substrate stably, a glass composition is required to have high thermal stability (for example, a high glass transition temperature or a high strain point).
JP 2006-169107 A discloses an aluminosilicate glass composition that can be produced by a method other than the downdraw process. This aluminosilicate glass composition is substantially free of alkali metal oxides, and consists essentially of, in terms of mass %: 60 to 67% of SiO2; 16 to 23% of Al2O3; 0 to 15% of B2O3; 0 to 8% of MgO, 0 to 18% of CaO, 0 to 15% of SrO, and 0 to 21% of BaO. The total content of MgO, CaO, SrO, and BaO is 12 to 30%. This composition is not, however, suitable for the downdraw process. Furthermore, as shown in Examples, this composition has a high content of BaO, which is not desirable in view of its environmental load and production cost.
JP 3988209 B2 discloses a glass composition suitable for a glass substrate for an FPD. This glass composition is substantially free of alkali metal oxides, and can be formed by the float process. This composition is not, however, suitable for the downdraw process because it has a high devitrification temperature of 1250° C. as shown in Examples.
JP 2009-013049 A discloses a glass composition being free of alkali metal oxides, As2O3, and Sb2O3, containing, in terms of mol %, 55 to 75% of SiO2, 7 to 15% of Al2O3, 7 to 12% of B2O3, 0 to 3% of MgO, 7 to 12% of CaO, 0 to 5% of SrO, 0 to 2% of BaO, 0 to 5% of ZnO, and 0.01 to 1% of SnO2, having a liquidus viscosity of 105.2dPa·s or higher, and having a temperature of 1550° C. or lower at a high temperature viscosity of 102.5dPa·s.
BaO, which is one of the glass components, is known to have effects of suppressing the phase separation of glass, improving the meltability, and decreasing the devitrification temperature (see paragraph 0023 of JP 3988209 B2). However, BaO has a high environmental load and its raw material is expensive, which results in an increase in the production cost of a glass substrate. Therefore, glass compositions substantially free of BaO are needed. SUMMARY OF THE INVENTION
It is an object of the present invention to provide a glass composition being suitable for an FPD glass substrate, having high thermal stability, being substantially free of BaO but having a low devitrification temperature, and being suitable for the production of a glass substrate by the downdraw process.
The glass composition of the present invention contains, in terms of mass %: 54 to 62% of SiO2; 4 to 11% of B2O3; 15 to 20% of Al2O3; 2 to 5% of MgO; 0 to 7% of CaO; 0 to 13.5% of SrO; 0 to 1% of K2O; 0 to 1% of SnO2; and 0 to 0.2% of Fe2O3. This glass composition is substantially free of BaO, the total content of alkaline earth metal oxides (MgO+CaO+SrO) is 10 to 18.5 mass %, and the devitrification temperature of the glass composition is 1200° C. or lower.
In the production method of the present invention, a melt of the glass composition of the present invention is formed into a glass substrate for an FPD by a downdraw process.
The glass composition of the present invention is suitable for a glass substrate for an FPD. This glass composition has high thermal stability. This glass composition has a low devitrification temperature even though it is substantially free of BaO. This glass composition is suitable for the production of a glass substrate by the downdraw process. BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a graph showing the dependence of the devitrification temperature of the glass composition of the present invention on the balance between the content of CaO and the content of SrO. In FIG. 1, the devitrification temperature is plotted as a function of the percentage (CaO/ROs) of CaO in all the alkaline earth metal oxides (ROs).
FIG. 2 is a graph showing the dependence of the devitrification temperature of the glass composition of the present invention on the balance between the content of CaO and the content of SrO. In FIG. 2, the devitrification temperature is plotted as a function of the percentage (SrO/ROs) of SrO in all the alkaline earth metal oxides (ROs).
FIG. 5 is a graph showing the linear thermal expansion coefficient of the glass composition of the present invention as a function of the content of K2O.
The reasons for determining the composition of the glass composition of the present invention are described below. In the following description, the unit “%” which expresses the contents of the components denotes “mass %”.
SiO2is a component for forming a glass skeleton, and has an effect of increasing the chemical durability and heat resistance of the glass. When the content of SiO2is less than 54%, such an effect cannot be obtained sufficiently. On the other hand, when the content of SiO2is more than 62%, the devitrification temperature of the glass increases. Furthermore, the melt viscosity of the glass increases as the meltability thereof deteriorates, which makes it difficult to form a glass substrate by the downdrawn process. Therefore, the SiO2content is 54% or more, preferably 55.5% or more, and more preferably 56.5% or more, focusing on its lower limit. The SiO2content is 62% or less, preferably 60% or less, and further preferably less than 58.4%, focusing on its upper limit. The content of SiO2is 54% or more and 62% or less, preferably 55.5% or more and 60% or less, and more preferably 56.5% or more and 58.4% or less.
B2O3is a component for decreasing the viscosity of glass and promoting the melting and refining of the glass. When the Content of B2O3is Less than 4%, the meltability of the glass deteriorates, which makes it difficult to form a glass substrate by the downdraw process. On the other hand, when the content of B2O3is more than 11%, the volatilization of B2O3from the surface of the molten glass increases, which makes it difficult to homogenize the glass. Therefore, the B2O3content is 4% or more, preferably 7% or more, and more preferably 8% or more, focusing on its lower limit. The B2O3content is 11% or less, and more preferably 10% or less, focusing on its upper limit. The content of B2O3is 4% or more and 11% or less, preferably 7% or more and 11% or less, and more preferably 8% or more and 10% or less.
Al2O3is a component for forming a glass skeleton, and has an effect of increasing the strain point of glass. A glass substrate used for a polysilicon (p-Si) LCD is required to have high thermal stability when a TFT circuit is formed thereon at a temperature of 500 to 600° C. Therefore, Al2O3, which has an effect of increasing the strain point of glass, is an important component for the glass composition of the present invention. When the content of Al2O3is less than 15%, the strain point of the glass decreases, and as a result, a glass composition suitable for a glass substrate for an FPD is not obtained. On the other hand, when the content of Al2O3is more than 20%, the acid resistance of the glass decreases, and the glass cannot withstand the acid treatment step in the production of FPDs, for example. Therefore, the Al2O3content is 15% or more, preferably 16% or more, and more preferably 18% or more, focusing on its lower limit. The Al2O3content is 20% or less, focusing on its upper limit. The content of Al2O3is 15% or more and 20% or less, preferably 16% or more and 20% or less, and more preferably 18% or more and 20% or less.
MgO is a component for decreasing the viscosity of glass and promoting the melting and refining of the glass. MgO further has an effect of decreasing the density of glass, and therefore it is effective in reducing the weight of the resulting glass and improving the meltability thereof. When the content of MgO is less than 2%, the meltability of the glass deteriorates, which makes it difficult to form a glass substrate by the downdraw process. On the other hand, when the content of MgO is more than 5%, the glass phase separation develops and thereby the acid resistance thereof decreases. As a result, the glass cannot withstand the acid treatment step in the production of FPDs, for example. Therefore, the MgO content is 2% or more, and preferably 3% or more, focusing on its lower limit. The MgO content is 5% or less, focusing on its upper limit. The content of MgO is 2% or more and 5% or less, and preferably 3% or more and 5% or less.
CaO is a component for decreasing the viscosity of glass and promoting the melting and refining of the glass. The glass composition of the present invention does not necessarily need to contain CaO, but preferably it contains CaO to improve the meltability of the glass and stabilize the production of a glass substrate by the downdraw process. Furthermore, a proper balance between the CaO content and the SrO content in the glass composition of the present invention allows the glass composition to have a lower devitrification temperature while maintaining high thermal stability thereof. In order to strike the balance, CaO needs to be contained in the glass composition. On the other hand, an excessively high content of CaO causes devitrification of glass. Therefore, such an excessively high content of CaO is not preferable. From these points of view, the CaO content is 0% or more, and preferably 0.2% or more, focusing on its lower limit. The CaO content is 7% or less, and preferably 4.5% or less, focusing on its upper limit. The content of CaO is 0% or more and 7% or less, and preferably 0.2% or more and 4.5% or less.
SrO is a component for decreasing the viscosity of glass and promoting the melting and refining of the glass. The glass composition of the present invention does not necessarily need to contain SrO, but preferably it contains SrO to improve the meltability of the glass and stabilize the production of a glass substrate by the downdraw process. Furthermore, in order to strike the balance between the CaO content and the SrO content, SrO needs to be contained in the glass composition. Moreover, the glass composition of the present invention is substantially free of BaO. From this point of view, too, SrO preferably is added. On the other hand, an excessively high content of SrO decreases the acid resistance of the glass, and as a result, the glass cannot withstand the acid treatment step in the production of FPDs, for example. From these points of view, the SrO content is 0% or more, and preferably 5% or more, focusing on its lower limit. The SrO content is 13.5% or less, preferably 12% or less, and more preferably 11.5% or less, focusing on its upper limit. The content of SrO is 0% or more and 13.5% or less, preferably 0% or more and 12% or less, and more preferably 5% or more and 11.5% or less.
The glass composition of the present invention is substantially free of BaO. Therefore, the glass composition of the present invention has a low environmental load and its production cost is low.
In the present description, “substantially free” means that a trace amount of impurities that have been inevitably mixed during the industrial production of the glass composition, such as impurities derived from the raw materials, may be contained. Specifically, “substantially free” is defined as the content of less than 0.5%, preferably less than 0.3%, and more preferably less than 0.1%.
An alkaline earth metal oxide RO (where R is Mg, Ca, or Sr) is a component having an effect on the melt viscosity of glass. When the total content of ROs (MgO+CaO+SrO) is less than 10%, the meltability of the glass decreases, which makes it difficult to form a glass substrate by the downdraw process. On the other hand, when the total content thereof is more than 18.5%, the acid resistance of the glass decreases, and the glass cannot withstand the acid treatment step in the production of FDPs, for example. Therefore, the total content of ROs is 10% or more, and preferably 12% or more, focusing on its lower limit. The total content of ROs is 18.5% or less, and preferably 16% or less, focusing on its upper limit. The total content of ROs is 10% or more and 18.5% or less, preferably 10% or more and 16% or less, and more preferably 12% or more and 16% or less.
In the glass composition of the present invention, it is preferable that the mass ratio Y1/X of the MgO content Y1to the total content X of ROs be 0.2 to 0.3, the mass ratio Y2/X of the CaO content Y2to the total content X be 0.01 to 0.3, and the mass ratio Y3/X of the SrO content Y3to the total content X be 0.4 to 0.74. In this case, a glass composition having a lower devitrification temperature while maintaining high thermal stability is obtained.
Specifically, a balance is kept between the percentage of CaO in all the ROs and the percentage of SrO therein while the percentage of MgO in all the ROs is maintained in a fixed range. As a result, a glass composition having a lower devitrification temperature while maintaining high thermal stability is obtained. FIG. 1 and FIG. 2 show the dependence of the devitrification temperature of the glass composition of the present invention on the balance between the percentage of CaO and the percentage of SrO in all the ROs. In this dependence, the percentage of MgO in all the ROs is almost constant. The horizontal axis of FIG. 1 indicates the mass ratio Y2/X of the CaO content Y2to the total content X of ROs (that is, the percentage CaO/ROs of CaO in all the ROs). The horizontal axis of FIG. 2 indicates the mass ratio Y3/X of the SrO content Y3to the total content X of ROs (that is, the percentage SrO/ROs of SrO in all the ROs). Specific glass compositions are shown in Table 1 below.
As shown in FIG. 1 and FIG. 2, the devitrification temperature of the glass is lowered when the mass ratio Y1/X of MgO to ROs is 0.2 to 0.3, the mass ratio Y2/X of CaO to ROs is 0.01 to 0.3, and the mass ratio Y3/X of SrO to ROs is 0.4 to 0.74. The devitrification temperature of the glass is lowered further when the mass ratio Y1/X of MgO to ROs is 0.2 to 0.3 and the mass ratio Y2/X of CaO to ROs is 0.05 to 0.23. Likewise, the devitrification temperature of the glass is lowered further when the mass ratio Y1/X of MgO to ROs is 0.2 to 0.3 and the mass ratio Y3/X of SrO to ROs is 0.48 to 0.70. Furthermore, the devitrification temperature of the glass shows a relative minimum value in the above ranges. In the glass compositions shown in Table 1, particularly low devitrification temperatures are obtained when the mass ratio Y2/X is 0.07 to 0.13 and/or the mass ratio Y3/X is 0.60 to 0.68, although the devitrification temperature also depends on the contents of the other components.
The glass composition of the present invention may contain K2O to adjust the thermal properties of the glass. The content of K2O is 0% or more and 1% or less.
The present inventors have found that there are special relationships between the K2O content, and the devitrification temperature, the glass transition temperature (Tg) and the linear thermal expansion coefficient of the glass composition of the present invention. FIG. 3 to FIG. 5 show the dependence of the devitrification temperature, that of the Tg, and that of the linear thermal expansion coefficient of the glass composition of the present invention, on the K2O content. In these dependences, the contents of the components other than K2O are almost constant. Specific glass compositions are shown in Table 2 below.
As shown in FIG. 3, in the glass composition of the present invention, the addition of 0.1% or more of K2O to the composition brings about a tendency for the devitrification temperature to drop sharply. Meanwhile, when the K2O content exceeds 1%, the decreasing tendency of the devitrification temperature is not seen. From this point of view, the content of K2O preferably is 0.1% or more and 1% or less. A low devitrification temperature is required in order to produce a glass substrate by the downdraw process.
On the other hand, it is desirable that a glass substrate for an FPD have stable physical properties, that is, high thermal stability, even during the formation of a TFT circuit thereon. As the Tg increases and the linear thermal expansion coefficient decreases, the thermal stability of the glass is enhanced. FIG. 4 shows that when the content of K2O is 0.5% or more, the Tg drops sharply. FIG. 5 shows that when the content of K2O is more than 0.5%, the linear thermal expansion coefficient rises sharply. That is, more preferably, the content of K2O is 0.1% or more and less than 0.5%. In this case, a better balance among the devitrification temperature, Tg, and linear thermal expansion coefficient is achieved.
There is a correlation between the glass transition temperature Tg and the above-mentioned strain point, and the approximate value of the strain point can be calculated from the Tg value. Normally, the higher the glass transition temperature Tg is, the higher the strain point is.
SnO2is a Component Having an Effect of Causing Molten Glass at High temperatures to produce gas by the valence change of Sn. Such a component can be used for refining glass. Conventionally, As2O3, Sb2O3, etc. are used for refining glass. With a recent growing awareness of environmental issues, there has been a demand for glass compositions not containing these materials as refining agents. SnO2has a low environmental load and a high refining effect. Therefore, preferably, the glass composition of the present invention contains SnO2. SnO2is, however, a component that causes glass to devitrify easily, and therefore, care should be taken to prevent the content of SnO2from increasing excessively. From these points of view, the SnO2content is 0% or more, and preferably 0.1% or more, focusing on its lower limit. The SnO2content is 1% or less, and preferably 0.5% or less, focusing on its upper limit. The content of SnO2is 0% or more and 1% or less, preferably 0.1% or more and 1% or less, and more preferably 0.1% or more and 0.5% or less.
Fe2O3is a component having effects of refining and coloring glass. In Order to use the glass composition of the present invention as a glass substrate for an FPD, it is desired that the content of Fe2O3be in the range where the refining effect is enhanced but the quality of the FPD glass substrate does not deteriorate. From this point of view, the Fe2O3content is 0% or more, and preferably 0.05% or more, focusing on its lower limit. The Fe2O3content is 0.2% or less, preferably 0.15% or less, and more preferably 0.1% or less, focusing on its upper limit. The content of Fe2O3is 0% or more and 0.2% or less, preferably 0.05% or more and 0.2% or less, more preferably 0.05% or more and 0.15% or less, and further preferably 0.05% or more and 0.1% or less.
The glass composition of the present invention may contain other components, such as Li2O, Na2O, TiO2, Cl, SO3, and ZnO, as refining agents or as components for adjusting the physical properties, in addition to the above-mentioned components, as long as the total content of the other components is in the range of 0.5% or less.
The glass composition of the present invention may consist essentially of the above-mentioned components, specifically, SiO2, B2O3, Al2O3, MgO, CaO, SrO, K2O, SnO2, Fe2O3, other components added for the purpose of adjusting the physical properties, and refining agents. In this case, the glass composition of the present invention is substantially free of components other than the above-mentioned components. In this description, “consists essentially of a set of components X” means that a glass composition referred to is substantially free of components other than the set of components X.
Specifically, the glass composition of the present invention may be a glass composition consisting essentially of, in terms of mass %; 54 to 62% of SiO2; 4 to 11% of B2O3; 15 to 20% of Al2O3; 2 to 5% of MgO; 0 to 7% of CaO; 0 to 13.5% of SrO; 0 to 1% of K2O; 0 to 1% of SnO2; and 0 to 0.2% of Fe2O3. In this glass composition, the total content of alkaline earth metal oxides (MgO+CaO+SrO) is 10 to 18.5%, and the devitrification temperature is 1200° C. or lower.
Preferably, the glass composition of the present invention is a glass composition in which the content of SiO2is 55.5% or more and 60% or less, the content of B2O3is 7% or more and 11% or less, the content of Al2O3is 16% or more and 20% or less, and the content of SrO is 0% or more and 12% or less. In this glass composition, the total content of the alkaline earth metal oxides (MgO+CaO+SrO) is 10% or more and 16% or less, and the devitrification temperature is 1160° C. or lower. This also applies to the case where the glass composition of the present invention is a glass composition consisting essentially of the above-mentioned components.
Preferably, the glass composition of the present invention is a glass composition in which the content of SiO2is 56.5% or more and less than 58.4%, the content of B2O3is 8% or more and 10% or less, the content of Al2O3is 18% or more and 20% or less, the content of MgO is 3% or more and 5% or less, the content of CaO is 0.2% or more and 4.5% or less, the content of SrO is 5% or more and 11.5% or less, and the content of K2O is 0.1% or more and 1% or less. In this glass composition, the total content of the alkaline earth metal oxides (MgO+CaO+SrO) is 12% or more and 16% or less, the mass ratio Y1/X of the MgO content Y1to the total content X of the alkaline earth metal oxides ROs is 0.2 to 0.3, the mass ratio Y2/X of the CaO content Y2to the total content X of ROs is 0.01 to 0.3, and the mass ratio Y3/X of the SrO content Y3to the total content X of ROs is 0.4 to 0.74, and the devitrification temperature is 1130° C. or lower. This also applies to the case where the glass composition of the present invention is a glass composition consisting essentially of the above-mentioned components.
The devitrification temperature of the glass composition of the present invention is 1200° C. or lower, and it is decreased to 1160° C. or lower or further to 1130° C. or lower in certain compositions. The devitrification temperature can be decreased to 1120° C. or lower, 1110° C. or lower, or 1100° C. or lower in some cases, as shown in the examples described below.
The Tg of the glass composition of the present invention is 710° C. or higher, for example, and it can be raised to 720° C. or higher in certain compositions. The glass composition of the present invention having such a high Tg has high thermal stability, and therefore, it is particularly suitable for use as a glass substrate for a polysilicon LCD.
The strain point of the glass composition of the present invention is 665° C. or higher, for example, and it can be raised to 670° C. or higher in certain compositions. The glass composition of the present invention having such a high strain point has high thermal stability, and therefore, it is particularly suitable for use as a glass substrate for a polysilicon LCD.
The thermal expansion coefficient of the glass composition of the present invention is 3×10−7/° C. to 38×10−7/° C., for example, in terms of a linear thermal expansion coefficient in the range of 50° C. to 300° C. The glass composition of the present invention having such a low thermal expansion coefficient has high thermal stability, and therefore, it is particularly suitable for use as a glass substrate for a polysilicon LCD.
As described above, in the downdraw process, molten glass is allowed to flow downward along the outer wall of a glass sheet forming apparatus. Therefore, the molten glass must have a viscosity that allows the glass to flow sufficiently. Specifically, if the viscosity of the molten glass is high during the forming step, its poor fluidity causes the glass to be more susceptible to forming defects.
Generally, in the forming step of the float process, the viscosity of the molten glass fed into a float bath is about 1000 Pa·s. In contrast, in the forming step of the downdraw process, the viscosity of the molten glass must be controlled at 4000 Pa·s to 50000 Pa·s.
In addition, in the downdraw process, the creep deformation of the forming member used in the glass sheet forming apparatus causes a problem. Therefore, preferably, the glass is formed at a lower temperature. For example, it is desired that the temperature of the molten glass be 1200° C. or lower when it is in contact with the forming member. Furthermore, in the downdraw process, if the temperature of a portion of the forming member that is in contact with the molten glass (for example, the above-mentioned outer wall) is equal to or lower than the devitrification temperature of the glass, a devitrified substance is produced at a portion of the glass that is in contact with the forming member. As a result, the forming quality deteriorates, or the forming operation itself cannot be performed. For these reasons, a glass to be produced by the downdraw process is required to have a particularly low devitrification temperature, compared to a glass to be produced by the float process.
The glass composition of the present invention has a particularly low devitrification temperature, and therefore is suitable for the downdraw process. Glass forming processes other than the downdraw process can be used as long as a desired glass article can be obtained.
The glass composition of the present invention is suitable for the production of glass substrates by the downdraw process, and it is suitable for the production of glass substrates for FPDs.
The glass substrate for an FPD of the present invention is composed of the glass composition of the present invention, and has physical properties corresponding to the physical properties of the glass composition of the present invention (for example, thermal properties such as strain point, Tg, and thermal expansion coefficient).
FIG. 6 shows an FPD glass substrate of the present invention. An FPD glass substrate 51 shown in FIG. 6 is composed of the glass composition of the present invention.
The FPD glass substrate of the present invention is suitable as a glass substrate for an LCD, in particular, as a glass substrate used for a polysilicon (p-Si) LCD on which a TFT circuit is formed at a temperature ranging from 500 to 600° C.
Typically, the FPD glass substrate of the present invention is formed by forming the glass composition of the present invention by the downdraw process. That is, the FPD glass substrate of the present invention is a glass substrate obtained by forming the glass composition of the present invention by the downdraw process. In the case where the FPD glass substrate of the present invention is a glass substrate obtained by the downdraw process, it has higher surface smoothness than glass substrates obtained by other processes, and therefore is particularly suitable as a glass substrate for an LCD.
The FPDs to which the FPD glass substrate of the present invention can be applied are not limited to any particular type. The FPDs are, for example, liquid crystal displays (LCDs), electroluminescent displays (ELDs), and field emission displays (FEDs). The FPDs to which the FPD glass substrate of the present invention can be applied are typically LCDs, and particularly preferably polysilicon LCDs.
The FPD of the present invention includes the FPD glass substrate of the present invention. FIG. 7 shows an example of the FPD of the present invention. An FPD 1 shown in FIG. 7 is a polysilicon active matrix LCD. The FPD 1 includes a pair of glass substrates 2 aand 2 b, and a pair of oriented films 3 aand 3 b. The oriented film 3 ais disposed in contact with the glass substrate 2 a, and the oriented film 3 bis disposed in contact with the glass substrate 2 b. Scanning lines 4, signal lines 5, and cells 6 each having a thin film transistor (TFT) are arranged on the glass substrate 2 b. Each of the cells 6 is formed in an intersection region that is surrounded by the scanning lines 4 and the signal lines 5 in plan view. In the FPD1, at least one substrate selected from the glass substrates 2 aand 2 bis the FPD glass substrate of the present invention. In other words, this at least one of the substrates is composed of the glass composition of the present invention. Preferably, the glass substrate 2 bon which the cells 6 each having a TFT are formed, among the glass substrates 2 aand 2 b, is the FPD glass substrate of the present invention.
The type and structure of the FPD of the present invention is not limited to those of the example shown in FIG. 7, as long as the FPD includes the FPD glass substrate of the present invention.
In the production method of the present invention, the melt of the glass composition of the present invention is formed into a glass substrate for an FPD by a downdraw process. An example of the production method of the present invention is described below with reference to FIG. 8.
First, glass raw materials are melted in a melting chamber 11 to form a melt of the glass composition of the present invention. The melt formed in the melting chamber 11 is delivered to a refining chamber 12 through a pipe 13 a, and refined in the refining chamber 12. The melt thus refined in the refining chamber 12 is delivered to a downdraw forming apparatus 14 through a pipe 13 b. The melt thus delivered to the forming apparatus 14 is allowed to flow over the upper edges of the apparatus 14 and flow downward along the wall surfaces (the front wall surface and the back wall surface in FIG. 7) of the apparatus 14. These wall surfaces meet at the lower end of the apparatus 14, and two streams of the melt flowing downward along the wall surfaces are fused together at the lower end of the apparatus 14. Thus, a single glass ribbon 15 is formed. The glass ribbon 15 thus obtained is cooled, and then cut into pieces of a desired size. Thus, glass substrates for FPDs are obtained.
The details of the production method of the present invention is not limited to any particular ones as long as the melt of the glass composition of the present invention is formed into a glass substrate for an FPD by the downdraw process. The structure of the apparatus used for the production method of the present invention, that of the downdraw forming apparatus in particular, also is not limited to any particular one. EXAMPLES
First, glass raw material batches were prepared so that glass compositions of Examples and Comparative Examples shown in Tables 3 to 7 were obtained from the batches. As the glass raw materials, silica, boracic anhydride, alumina, basic magnesium carbonate, calcium carbonate, strontium carbonate, potassium carbonate, tin oxide, and iron oxide were used.
Next, each of the raw material batches thus prepared was put into a platinum crucible and allowed to stand for 2 hours in an electric furnace at a setting temperature of 1550° C., and then allowed to stand for another 2 hours in the electric furnace at a setting temperature of 1620° C. Thus, molten glass was obtained. Next, the molten glass thus obtained was poured on an iron plate to obtain a glass block. The glass block was placed in an electric furnace set at 750° C., and allowed to stand for 30 minutes, followed by cooling to 550° C. for 2 hours. Then, the electric furnace, in which the glass block was placed, was turned off so that the glass block was cooled to room temperature. Thus, a glass sample was obtained.
The devitrification temperature of the glass sample was measured in the following manner. First, the glass sample was ground in a mortar to obtain glass particles. The glass particles were subjected to sieving, and particles that passed through a sieve with a 2380 μm mesh size but did not pass through a sieve with a 1000 μm mesh size were gathered. Next, the gathered glass particles were cleaned ultrasonically in ethanol, and dried. Thus, a measurement sample was obtained. Next, 25 g of the measurement sample thus obtained was placed in a platinum boat of 12 mm in width and 200 mm in length. The platinum boat was placed in a gradient heating furnace and allowed to stand for 24 hours. Next, the platinum boat containing the glass was taken out of the furnace and cooled to room temperature. Then, crystals formed in the glass (devitrification) were observed with an optical microscope. This experiment was carried out in various ranges of measurement temperatures of the furnace, and the highest temperature at which crystals were observed was defined as the devitrification temperature of the glass sample.
The linear thermal expansion coefficient of the glass sample was measured in the following manner. First, the glass sample was worked into a cy




 Ms.Josey
Ms.Josey 
 Ms.Josey
Ms.Josey